Large sample dose content uniformity test parametric and



- Slides: 24

Large sample dose content uniformity test: parametric and nonparametric (counting) Meiyu Shen, Ph. D Collaborators: Xiaoyu Dong, Ph. D. , Yi Tsong, Ph. D Office of Biostatistics, CDER, FDA * This presentation contains opinions of the authors that do not represent the official position of U. S. Food and Drug Administration 1

Outline • Purpose of uniformity of dosage unit • Harmonized USP dose content uniformity test with a small sample • Large n dose content uniformity test – EU methods • Option 1: Parametric method • Option 2: Nonparametric method (Counting method) – Two one-sided tolerance interval method • Comparison between the EU method and the two one-sided tolerance interval method • Conclusion 2

Uniformity of dosage unit • The purpose of uniformity of dosage unit – The degree of uniformity in the amount of the drug substance among dosage units. • Demonstrated by one of the follows – Dose content uniformity (focus here) • based on the assay of the individual content of drug substance(s) in a number of dosage units – Weight variation 3

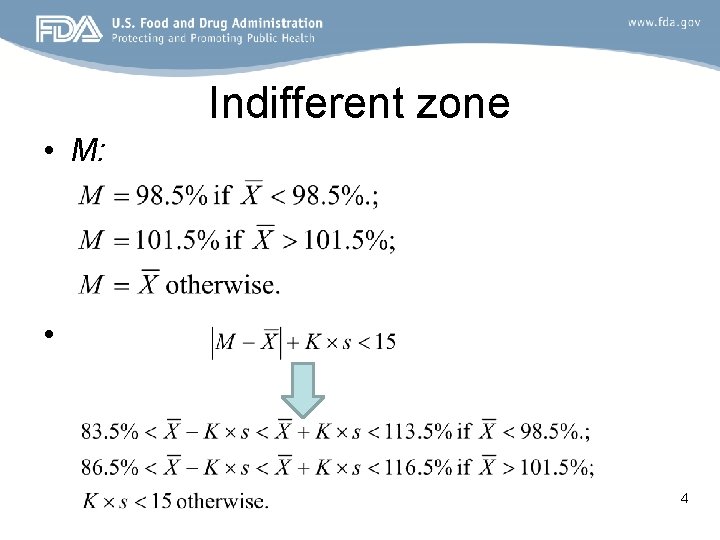
Indifferent zone • M: • 4

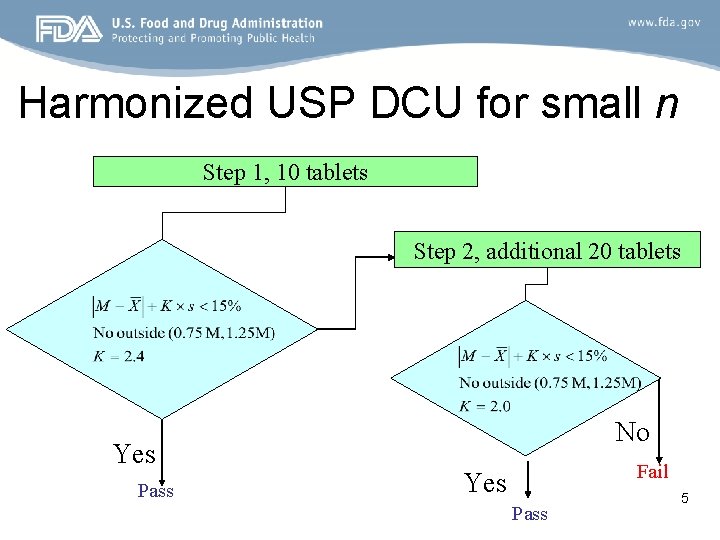
Harmonized USP DCU for small n Step 1, 10 tablets Step 2, additional 20 tablets Yes Pass No Fail Yes Pass 5

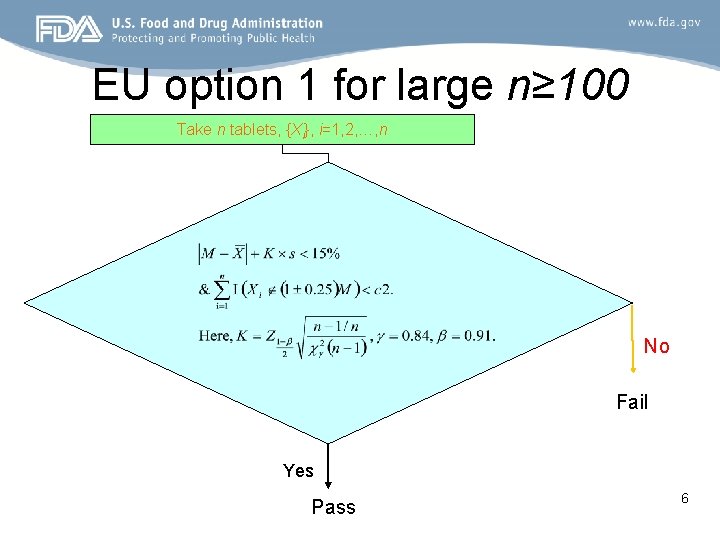
EU option 1 for large n≥ 100 Take n tablets, {Xi}, i=1, 2, …, n No Fail Yes Pass 6

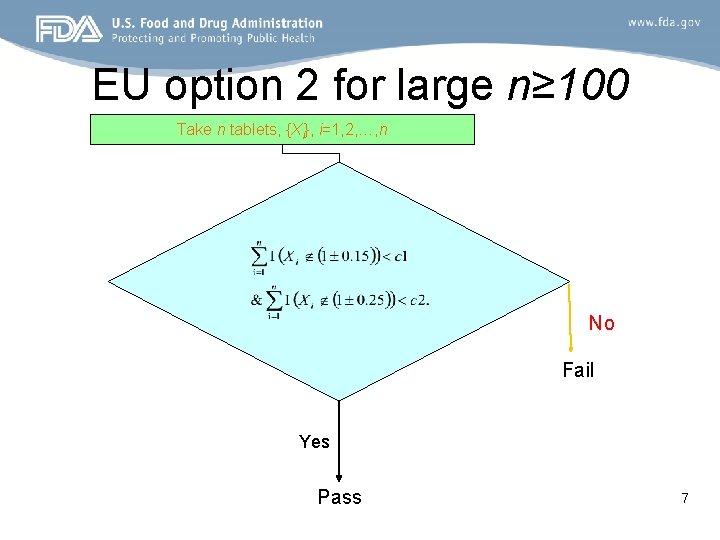
EU option 2 for large n≥ 100 Take n tablets, {Xi}, i=1, 2, …, n No Fail Yes Pass 7

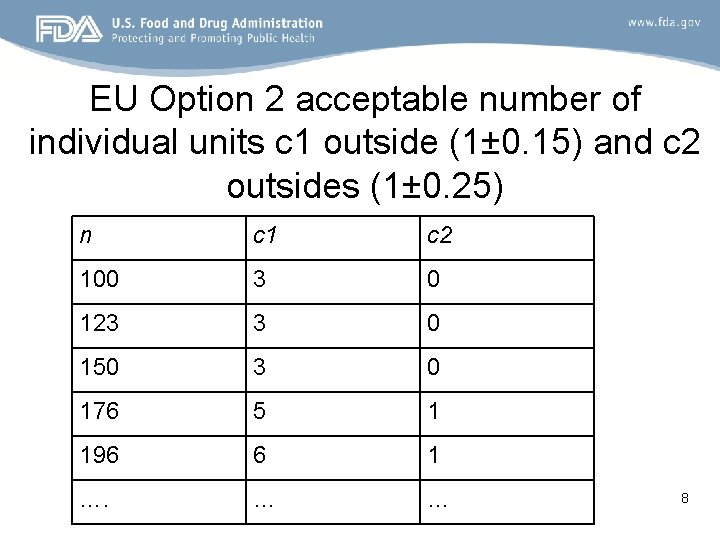
EU Option 2 acceptable number of individual units c 1 outside (1± 0. 15) and c 2 outsides (1± 0. 25) n c 1 c 2 100 3 0 123 3 0 150 3 0 176 5 1 196 6 1 …. … … 8

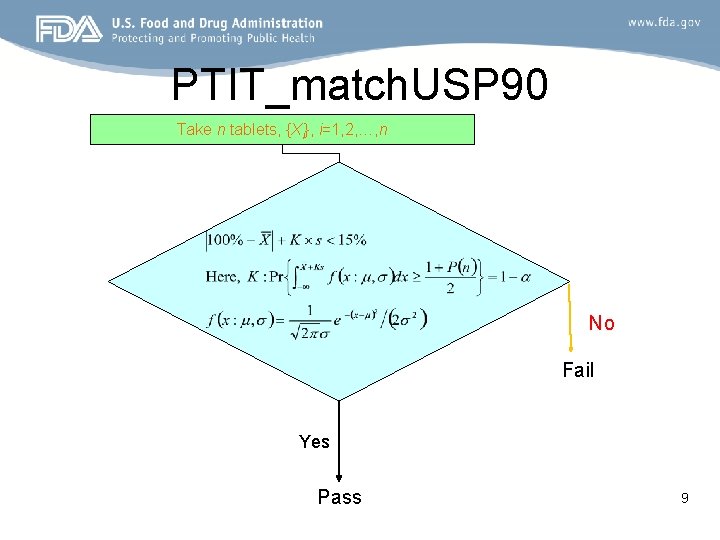
PTIT_match. USP 90 Take n tablets, {Xi}, i=1, 2, …, n No Fail Yes Pass 9

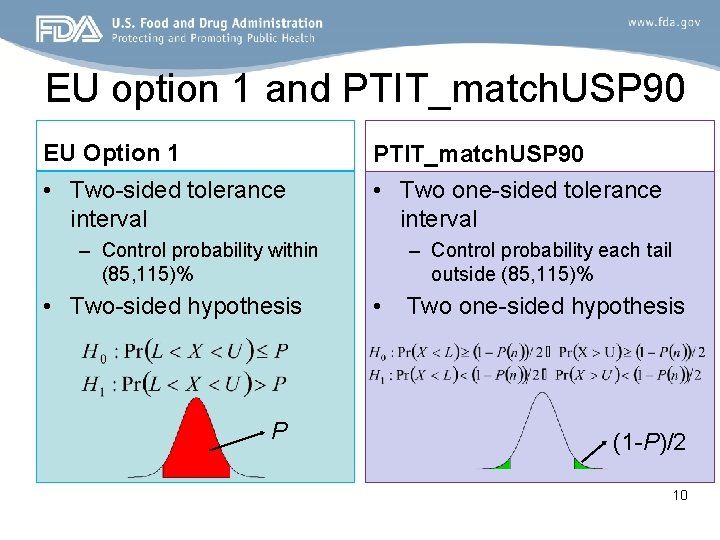
EU option 1 and PTIT_match. USP 90 EU Option 1 PTIT_match. USP 90 • Two-sided tolerance interval • Two one-sided tolerance interval – Control probability within (85, 115)% • Two-sided hypothesis P – Control probability each tail outside (85, 115)% • Two one-sided hypothesis (1 -P)/2 10

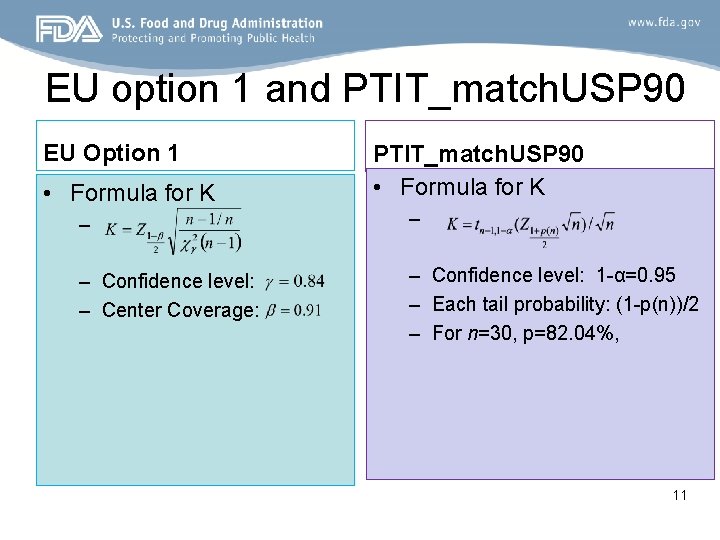
EU option 1 and PTIT_match. USP 90 EU Option 1 • Formula for K PTIT_match. USP 90 • Formula for K – – – Confidence level: – Center Coverage: – Confidence level: 1 -α=0. 95 – Each tail probability: (1 -p(n))/2 – For n=30, p=82. 04%, 11

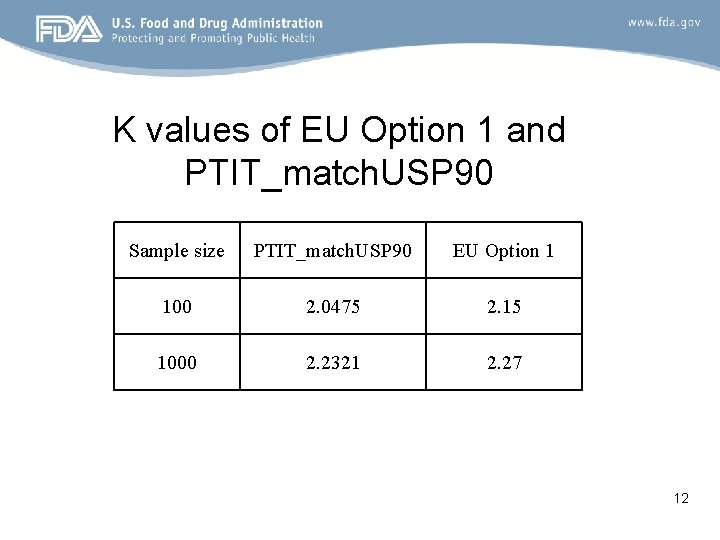
K values of EU Option 1 and PTIT_match. USP 90 Sample size PTIT_match. USP 90 EU Option 1 100 2. 0475 2. 15 1000 2. 2321 2. 27 12

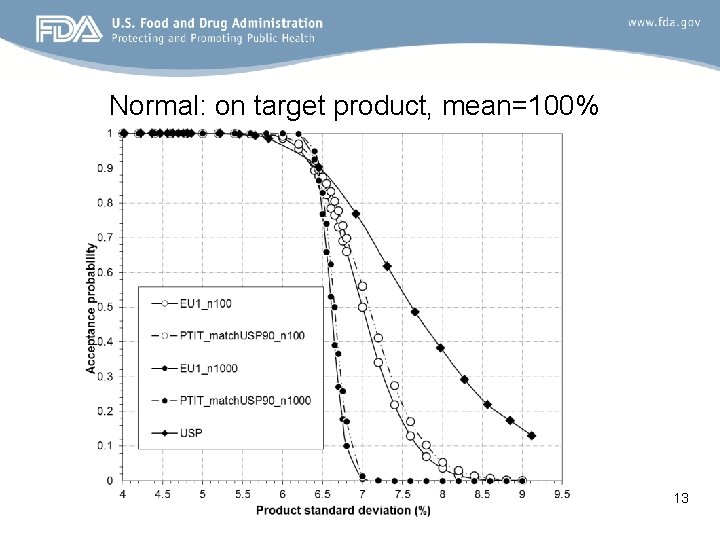
Normal: on target product, mean=100% 13

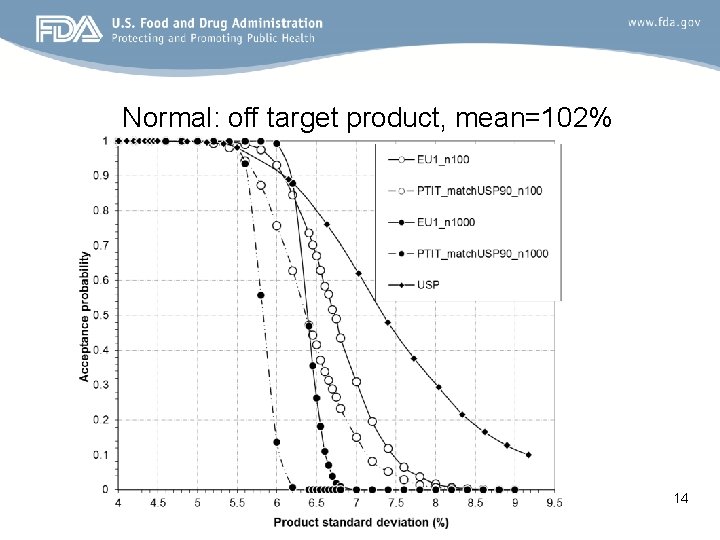
Normal: off target product, mean=102% 14

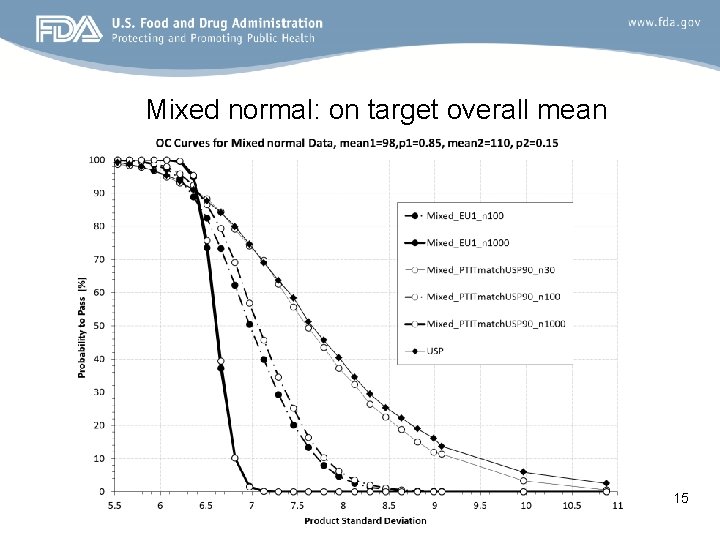
Mixed normal: on target overall mean 15

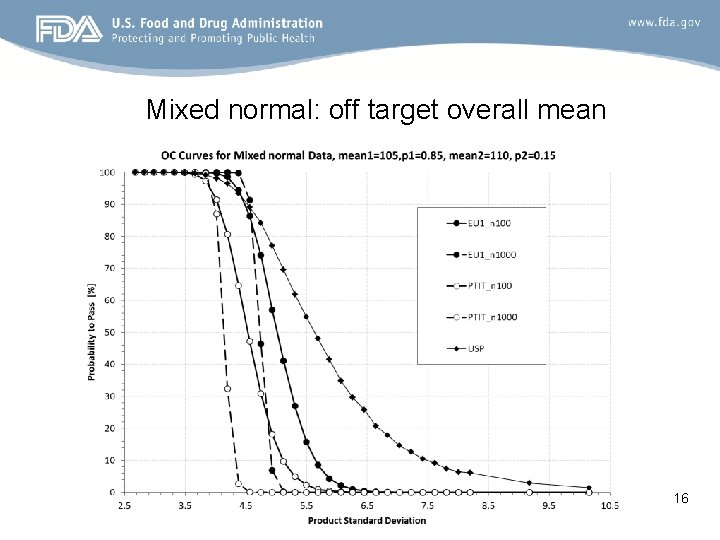
Mixed normal: off target overall mean 16

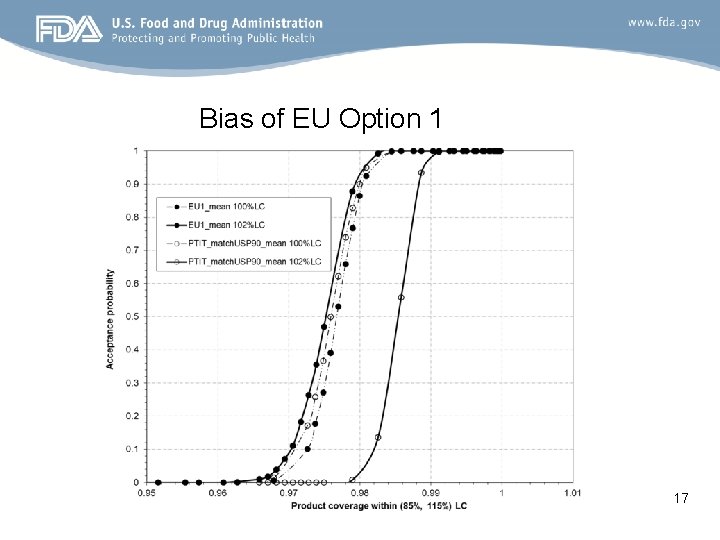
Bias of EU Option 1 17

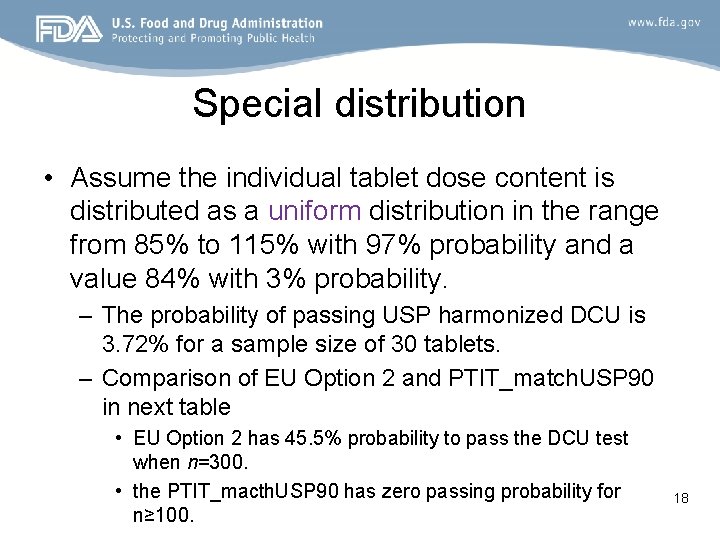
Special distribution • Assume the individual tablet dose content is distributed as a uniform distribution in the range from 85% to 115% with 97% probability and a value 84% with 3% probability. – The probability of passing USP harmonized DCU is 3. 72% for a sample size of 30 tablets. – Comparison of EU Option 2 and PTIT_match. USP 90 in next table • EU Option 2 has 45. 5% probability to pass the DCU test when n=300. • the PTIT_macth. USP 90 has zero passing probability for n≥ 100. 18

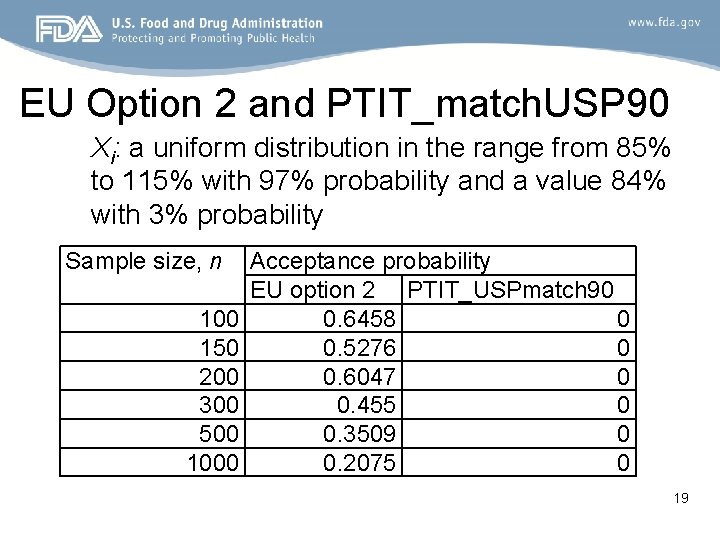
EU Option 2 and PTIT_match. USP 90 Xi: a uniform distribution in the range from 85% to 115% with 97% probability and a value 84% with 3% probability Sample size, n 100 150 200 300 500 1000 Acceptance probability EU option 2 PTIT_USPmatch 90 0. 6458 0 0. 5276 0 0. 6047 0 0. 455 0 0. 3509 0 0. 2075 0 19

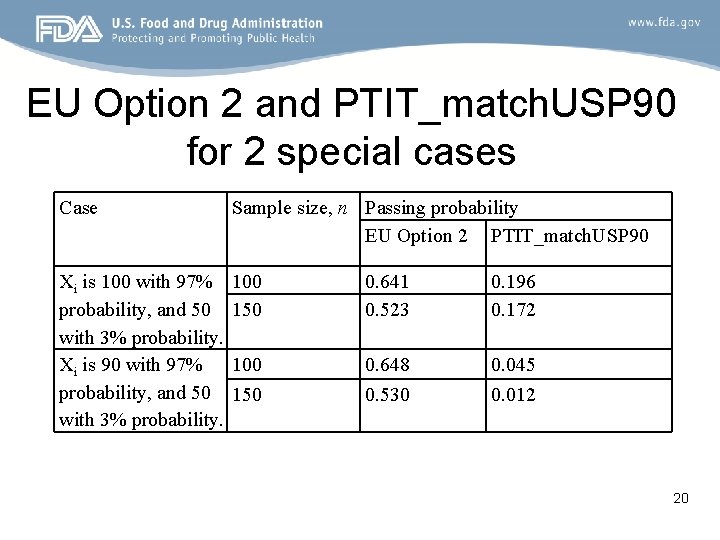
EU Option 2 and PTIT_match. USP 90 for 2 special cases Case Sample size, n Passing probability EU Option 2 PTIT_match. USP 90 Xi is 100 with 97% probability, and 50 with 3% probability. Xi is 90 with 97% probability, and 50 with 3% probability. 100 150 0. 641 0. 523 0. 196 0. 172 100 150 0. 648 0. 530 0. 045 0. 012 20

Conclusion • A large difference in acceptance probability between EU option 1 and PTIT_match. USP 90 when the batch mean is off -target. – Larger passing probability for EU Option 1 than PTIT_match. USP 90 • No much difference in acceptance probability between EU option 1 and PTIT_match. USP 90 when the batch mean is on -target. • Bias of EU Option 1 – EU Option 1 has higher probability of passing the off-target product than that of passing the on-target mean product for a given coverage within (85%, 115%) 21

Conclusion (continued) • EU Option 2 – Issue with a large variability for a mixture of 97% probability of distributing uniformly with (85%, 115%) and 3% probability of being 84%) using a sample of 200 • 60% probability to pass EU Option 2 • 0% probability to pass PTIT_match. USP 90 • 3% probability to pass USP harmonized – Issue with a location shift of the mean product • The same probability to pass the EU Option 2 for 97% population with 100% content and 97% population with 90% content. • Off target product: 97% population with 90% content using a sample of 150. – >50% probability to pass the EU Option 2 – About 1% probability to pass PTIT_match. USP 90 22

References • • USP Pharmacopoeia 2015 European Pharmacopoeia 7. 7 European Pharmacopoeia 8. 1 Meiyu Shen, Yi Tsong, Xiaoyu Dong, Statistical Properties of Large Sample Tests for Dose Content Uniformity, Therapeutic Innovation & Regulatory Science, 2014, Vol. 48(5) 613 -622 • Meiyu Shen and Yi Tsong, Bias Of The United States Pharmacopeia Harmonized Test For Dose Content Uniformity, United States Pharmacopeia forum, January 2011 23

Thank you! 24