Language of Chemistry Molecules Monatomic 1 atom Molecules



























- Slides: 46

Language of Chemistry

Molecules • Monatomic = 1 atom

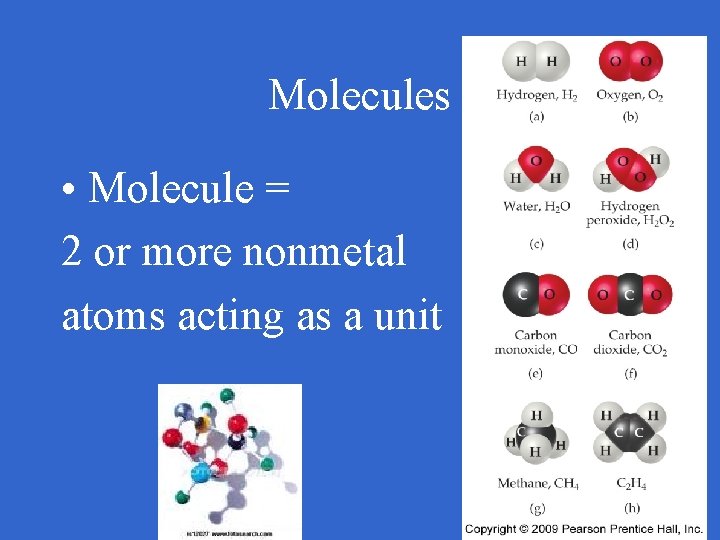
Molecules • Molecule = 2 or more nonmetal atoms acting as a unit

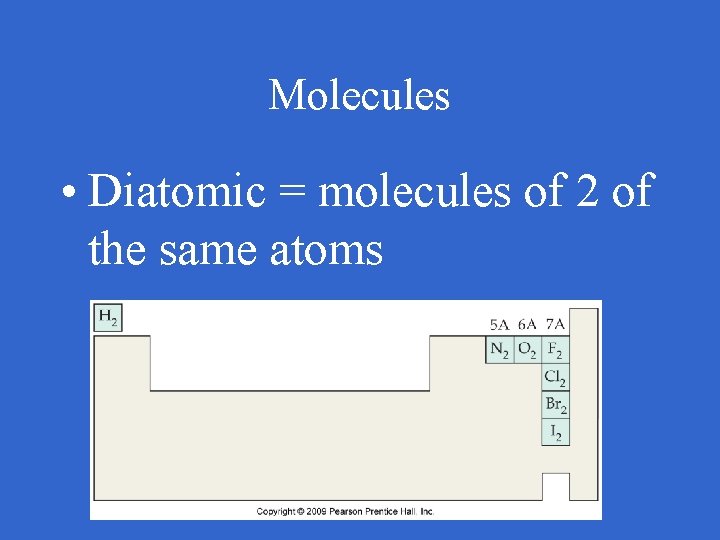
Molecules • Diatomic = molecules of 2 of the same atoms

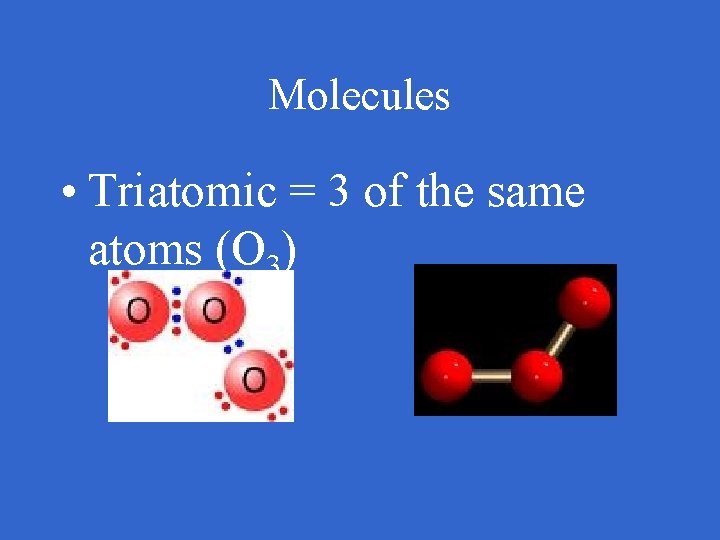
Molecules • Triatomic = 3 of the same atoms (O 3)

Molecular Compounds • • • Composed of molecules Low melting and boiling points Do not conduct electricity in H 2 O Gases or liquids at room temp 2 or more different nonmetals

Ions • Atoms or groups of atoms with a positive or negative charge • Form when atoms lose or gain electrons


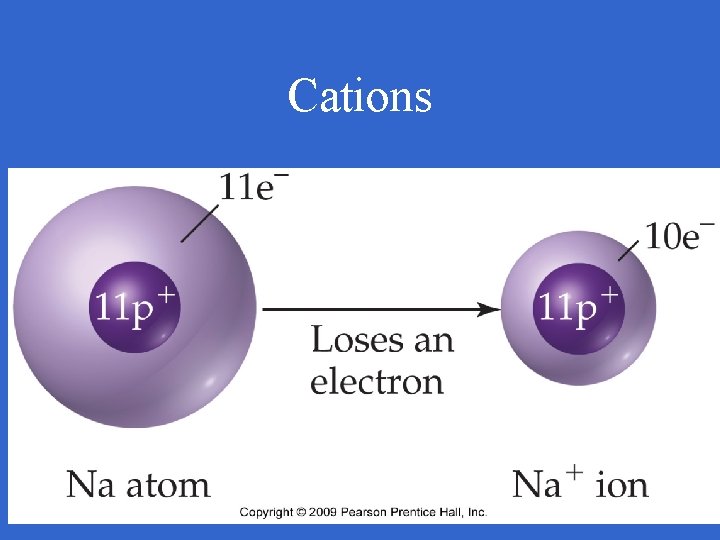
Cations • Any atom or group of atoms with a positive charge • It has lost electrons • Metals

Cations

Cations • Written with a symbol and charge; Na+ • Named by element; sodium ion

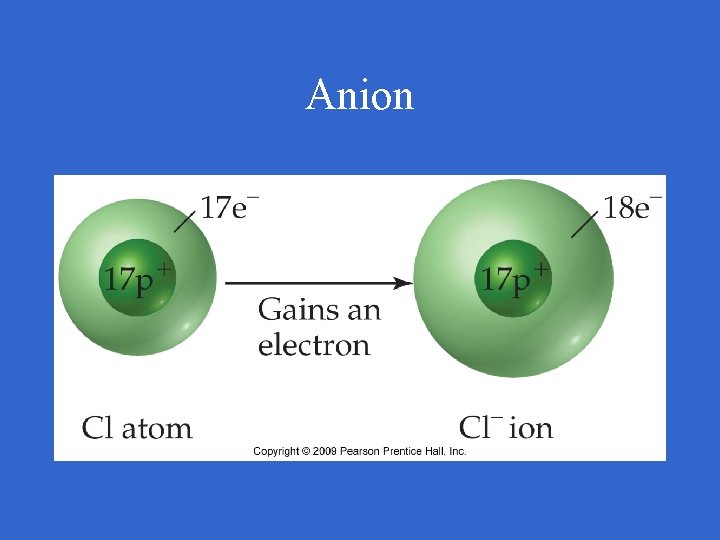
Anion • Atoms or groups of atoms with a negative charge • Gained electrons • Nonmetals

Anion

Anion • Written with symbol and charge; Cl • Named by elements name plus –ide; chloride ion

Ionic Compounds • Composed of ions; cations and anions; metal and nonmetal • Solid crystals at room temperature • High mp/ bp • Neutral • Na+ + Cl- Na. Cl • Conduct electricity In H 2 O

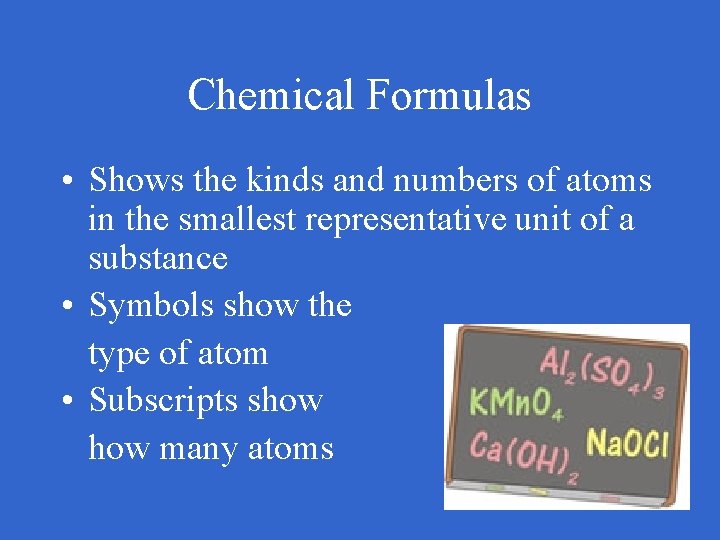
Chemical Formulas • Shows the kinds and numbers of atoms in the smallest representative unit of a substance • Symbols show the type of atom • Subscripts show many atoms

Summary • What does it mean to say an atom is neutral? • What happens to the charge of an atom if an electron is removed? Gained? • What is a molecule? • What is the difference between molecular and ionic compounds?

Dalton’s Laws

Law of Conservation of Mass • Atoms are neither created nor destroyed

Law of Definite Proportions • In any sample size of a compound, the mass of the elements are always in the same proportions

Law of Definite Proportions • If you take 100 g of Mg. S, you always obtain a ratio of 43. 13 g Mg / 56. 87 g S or 0. 7584: 1.

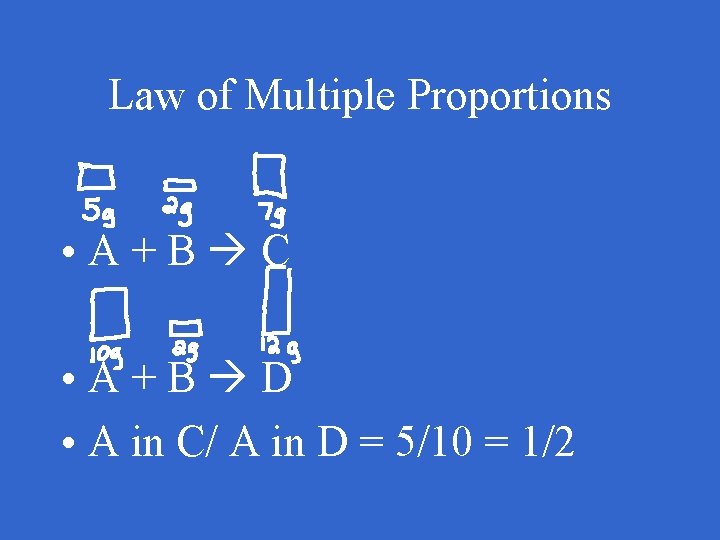
Law of Multiple Proportions • When any two elements form more than one compound, the different masses of one element that combine with the same mass of the other element are in small whole number ratios

Law of Multiple Proportions • A+B C • A+B D • A in C/ A in D = 5/10 = 1/2

Dalton’s Laws • Which law is illustrated below? • “In every sample of carbon monoxide, the mass ratio of C: O is 3: 4”

Dalton’s Laws • Which law is illustrated below? • “When C and O form CO and CO 2, the different masses of C that combine with the same mass of O is in a ratio of 2: 1”

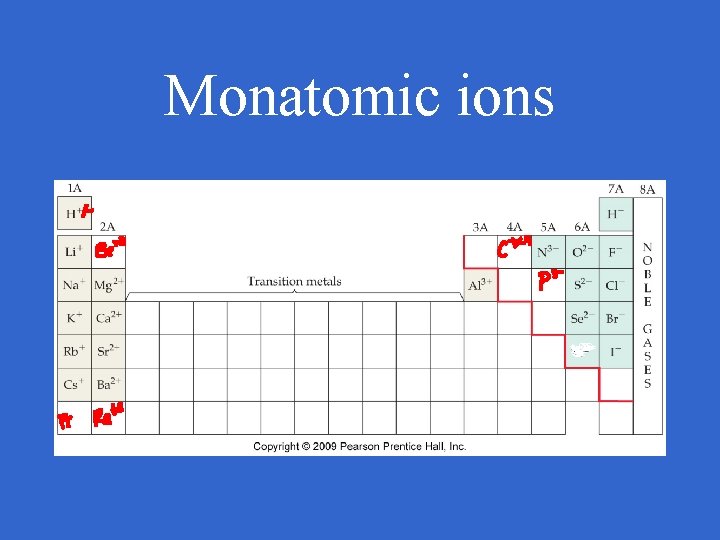
Monatomic ions • ions made of one type of atom

Monatomic ions • Metallic elements tend to lose electrons to form cations • For group A metals, the charge equals the group #

Monatomic ions • Nonmetallic elements tend to gain electrons to form anions • For group A nonmetals, the charge equals the group # - 8

Monatomic ions • Hydrogen is + or • Carbon makes +/- 4 • group 8 usually do not make ions

Monatomic ions

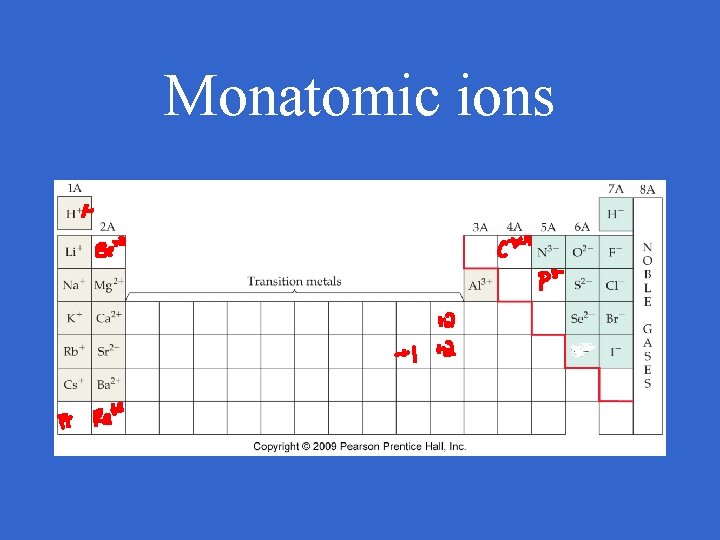
Monatomic ions • For all other metals (group B metals, others) there is often more than one possible charge

Monatomic ions • For group B and other metals, the classical system used to be used for naming

Monatomic ions • Now the stock system is used and a roman numeral is used to represent the charge or the charge can be determined by the formula

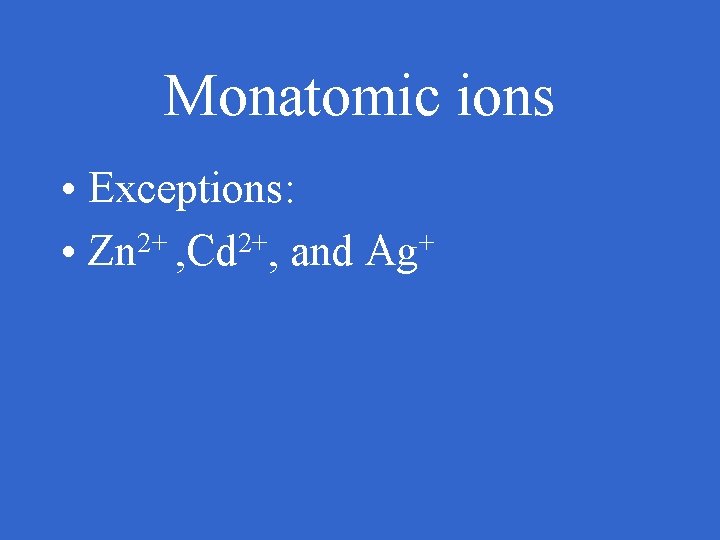
Monatomic ions • Exceptions: • Zn 2+ , Cd 2+, and Ag+

Monatomic ions

Polyatomic ions • tightly bound groups of atoms that act as a unit and carry a charge

Summary • What type of ions form from metals? Nonmetals? • What is the difference between Cu , Cu+, and Cu 2+? • What is an ionic compound?

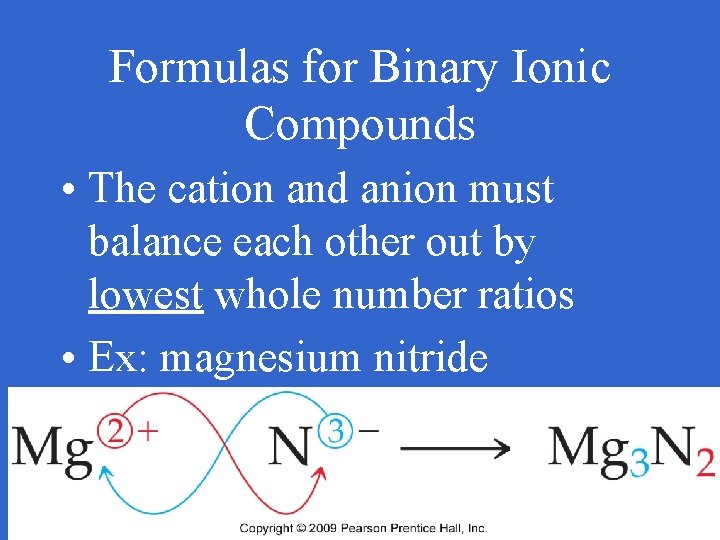
Formulas for Binary Ionic Compounds • The cation and anion must balance each other out by lowest whole number ratios • Ex: magnesium nitride

Names for Binary Ionic Compounds • Name the cation and the anion • If it is a metal with more than one charge, don’t forget the roman numeral

Ternary Ionic Compounds • Same rules as binary ionic compounds except you have a polyatomic ion

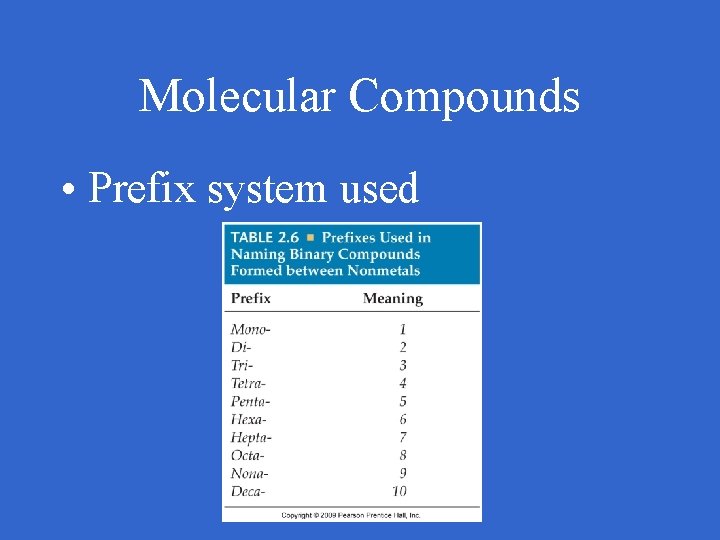
Molecular Compounds • Prefix system used

Molecular Compounds • Prefix system used

Molecular Compounds • Prefix 1 element 1 prefix 2 element 2 + -ide • Except mono is never used on element 1

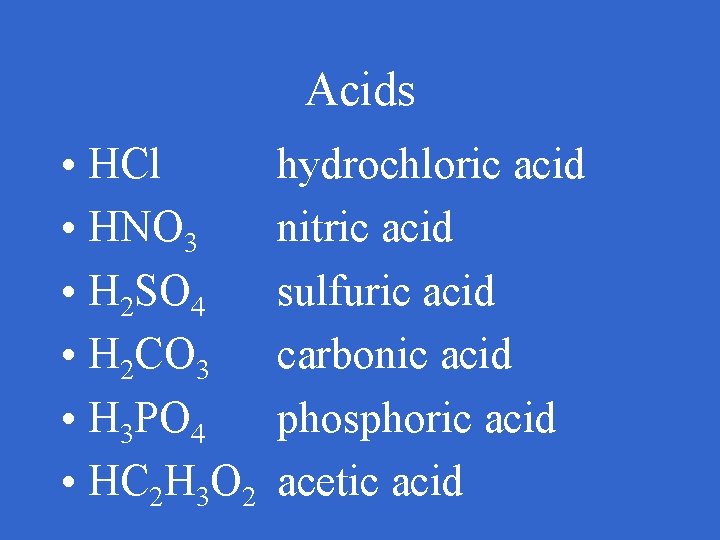
Acids

Acids • 6 common acids names and formulas to know

Acids • HCl • HNO 3 • H 2 SO 4 • H 2 CO 3 • H 3 PO 4 • HC 2 H 3 O 2 hydrochloric acid nitric acid sulfuric acid carbonic acid phosphoric acid acetic acid