Labs from Units 2 3 and 4 Guided

Labs from Units 2, 3, and 4: Guided Inquiry: Molarity, Set Up Lab Equipment, Begin Lab Set Up
Warm Up In your Warm Up Section, answer the following questions: • How many moles are in 45. 3 grams of aluminum oxide? • What can you to make a 1 Molar solution of it? TIME: 6 MINUTES WHEN DONE: Share out with your table partners

Agenda • • Finish Notes on Molarity, Dilution, and Titration Check Out Lab Equipment – Again Guided Inquiry: Molarity Set Up: Lab Notebook
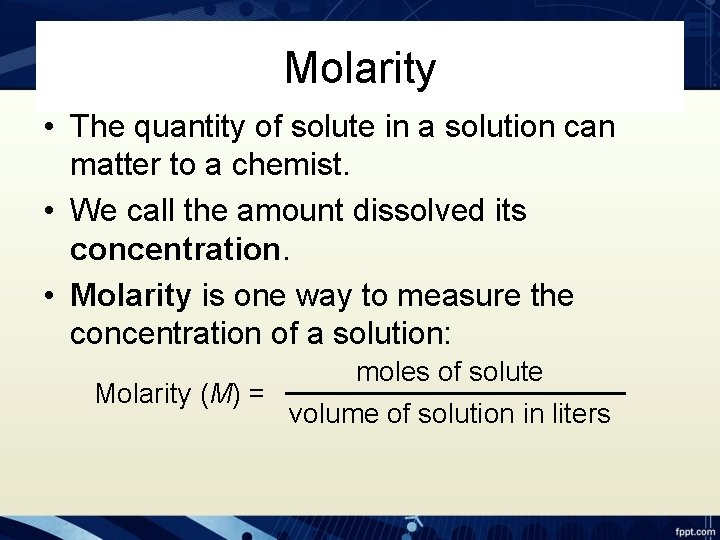
Molarity • The quantity of solute in a solution can matter to a chemist. • We call the amount dissolved its concentration. • Molarity is one way to measure the concentration of a solution: moles of solute Molarity (M) = volume of solution in liters

Mixing a Solution • To create a solution of a known MOLARITY, weigh out a known mass (and, therefore, number of moles) of the solute. • Then add solute to a volumetric flask, and add solvent to the line on the neck of the flask.

3 STEPS TO CALCULATING MOLARITY 1. Convert solute to moles (using molar mass) 2. Convert solvent to liters 3. Divide: moles of solute Liters of solvent
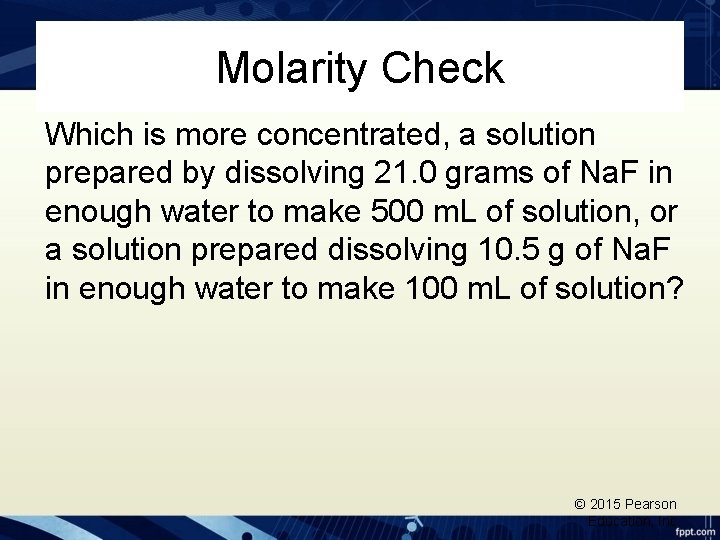
Molarity Check Which is more concentrated, a solution prepared by dissolving 21. 0 grams of Na. F in enough water to make 500 m. L of solution, or a solution prepared dissolving 10. 5 g of Na. F in enough water to make 100 m. L of solution? © 2015 Pearson Education, Inc.
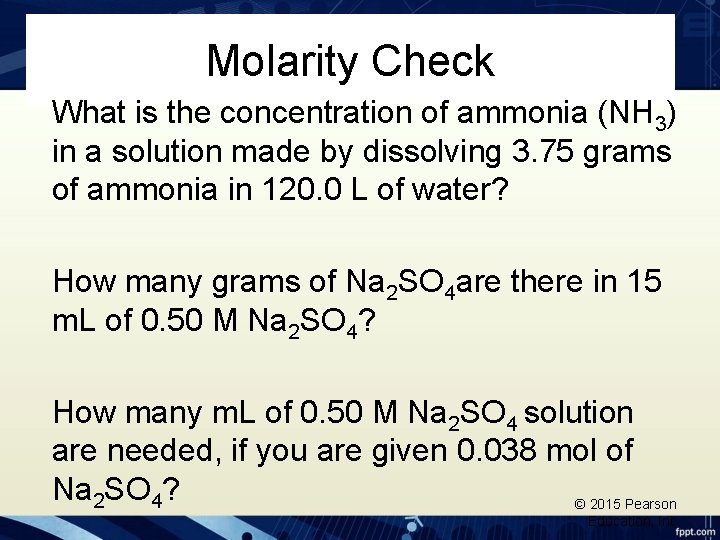
Molarity Check What is the concentration of ammonia (NH 3) in a solution made by dissolving 3. 75 grams of ammonia in 120. 0 L of water? How many grams of Na 2 SO 4 are there in 15 m. L of 0. 50 M Na 2 SO 4? How many m. L of 0. 50 M Na 2 SO 4 solution are needed, if you are given 0. 038 mol of Na 2 SO 4? © 2015 Pearson Education, Inc.
Molarity Check What is the molar concentration of K+ ions in a 0. 015 M solution of potassium carbonate? © 2015 Pearson Education, Inc.

Dilution • One can also dilute a more concentrated solution by – using a pipet to deliver a volume of the solution to a new volumetric flask, and – adding solvent to the line on the neck of the new flask.

Dilution The molarity of the new solution can be determined from the equation M 1 V 1 = M 2 V 2 , where M 1 and M 2 are the molarity of the concentrated and dilute solutions, respectively, and V 1 and V 2 are the volumes of the two solutions.

Dilution: Check You are given 250 grams of Copper II Chloride dissolved and you dissolve it in 360 m. L of water. You then notice that your solution is WAY TOO concentrated. You only need to make a 0. 050 M solution of Copper II Chloride, not the one you made. How much more water do you need to add to make 0. 05 M Copper II Chloride?

Dilution: Check What volume of a 1. 00 M stock solution of glucose must be used to make 500 m. L of a 1. 75 x 10 -2 M glucose solution? a) 1. 75 m. L b) 8. 75 m. L c) 48. 6 m. L d) 28570 m. L
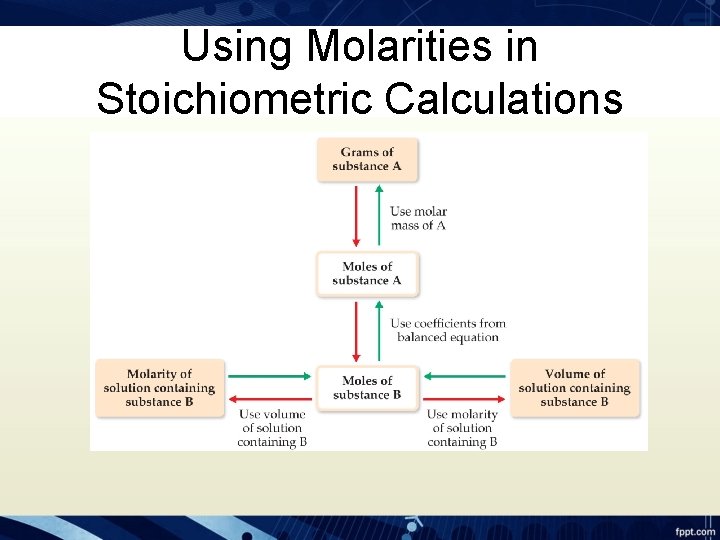
Using Molarities in Stoichiometric Calculations
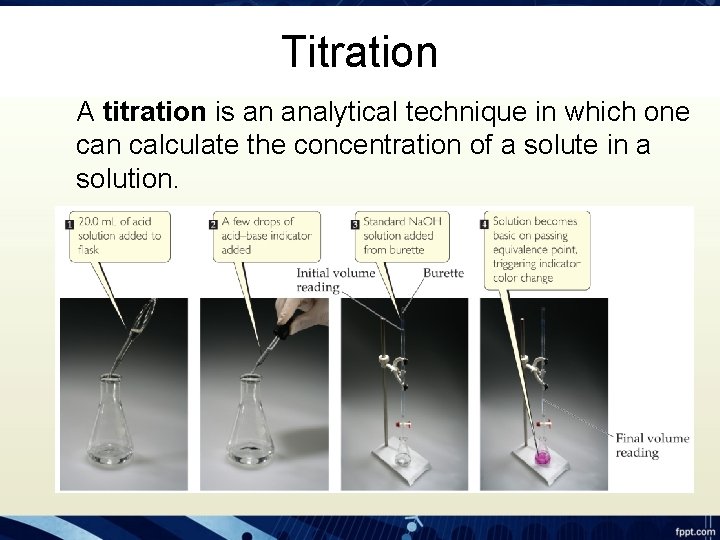
Titration A titration is an analytical technique in which one can calculate the concentration of a solute in a solution.
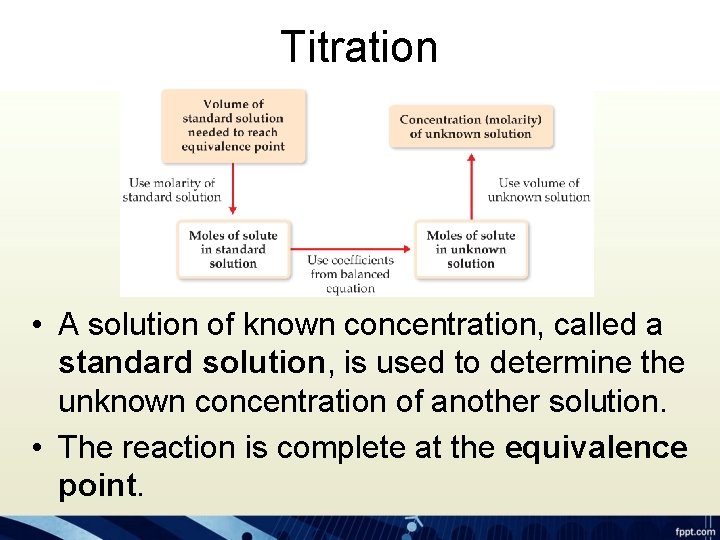
Titration • A solution of known concentration, called a standard solution, is used to determine the unknown concentration of another solution. • The reaction is complete at the equivalence point.

Lab Equipment FIND YOUR BINS: Spread Out to Other Tables FILL: ALL Bins with 2 -3 Paper Towels Each CHECK OUT: Lab Equipment IF YOU ARE MISSING ANY ITEM: Let me know/DO NOT check it off TIME: 11 MINUTES WHEN DONE: Work on Guided Inquiry: Molarity

Guided Inquiry: Molarity COMPLETE: Molarity Guided Inquiry WORK: Together USE: Notes and each other TIME: 15 MINUTES WHEN DONE: Turn into class box and begin reading pre-lab

SET UP LAB NOTEBOOK
- Slides: 19