Laboratory Surveillance of Sexually Transmitted Disease LA County

Laboratory Surveillance of Sexually Transmitted Disease LA County Dept. of Health Services Sexually Transmitted Disease Program Laboratory Surveillance Unit Chandra Higgins, MPH chiggins@ladhs. org
Reportable STDs in LA County • Reporting requirements • Dual reporting system • Forms

Legal STD Reporting Requirements in California Laboratory Health Provider • Syphilis • Chlamydia • Gonorrhea • • • Syphilis Chlamydia Gonorrhea Chancroid PID (Pelvic Inflammatory Disease) • NGU (Non-Gonococcal Urethritis)

Reporting Responsibilities Laboratory Health Provider • All positives • Report within one working day of identification • Known or suspect • Report syphilis within one working day of identification. All others within 7 calendar days.

Reporting Responsibilities Laboratory Health Provider • Patient name & limited demographics • Provider name, address, phone • Specimen accession, dates, test type & results • Patient name & full demographics • Diagnosis, date of diagnosis & onset • Name, address, & phone of reporter
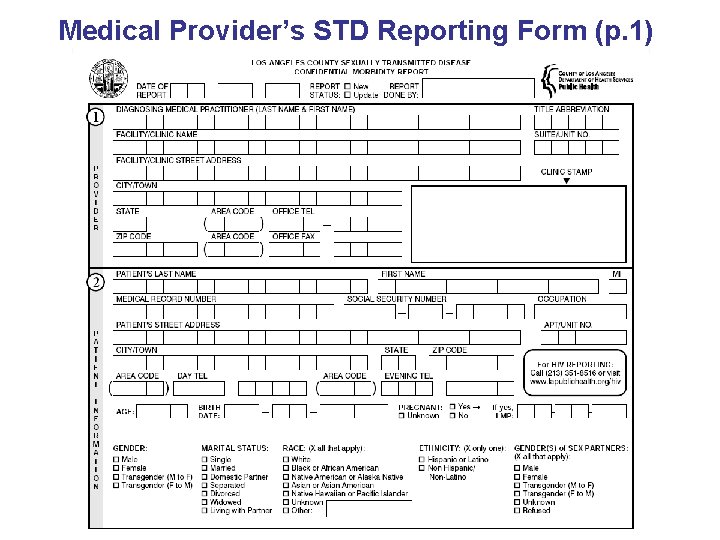
Medical Provider’s STD Reporting Form (p. 1)
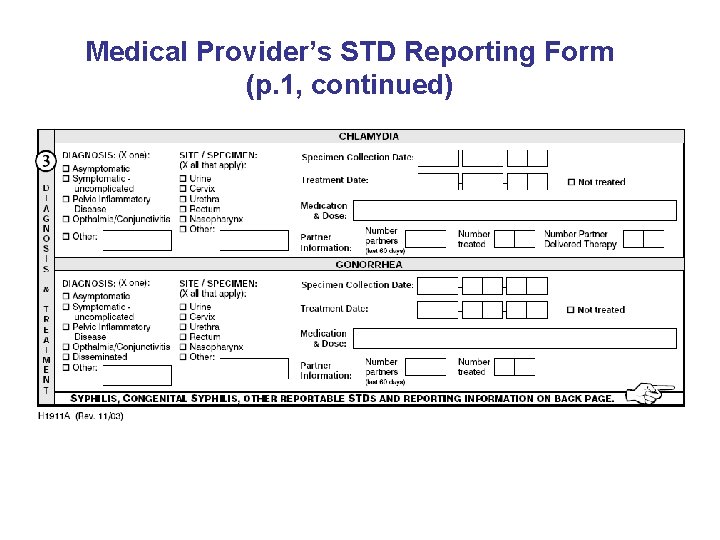
Medical Provider’s STD Reporting Form (p. 1, continued)
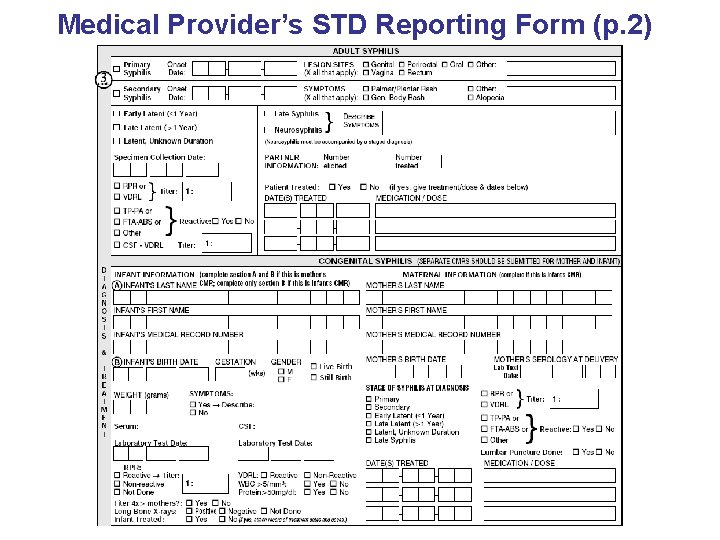
Medical Provider’s STD Reporting Form (p. 2)
Medical Provider’s STD Reporting Form (p. 2, continued)
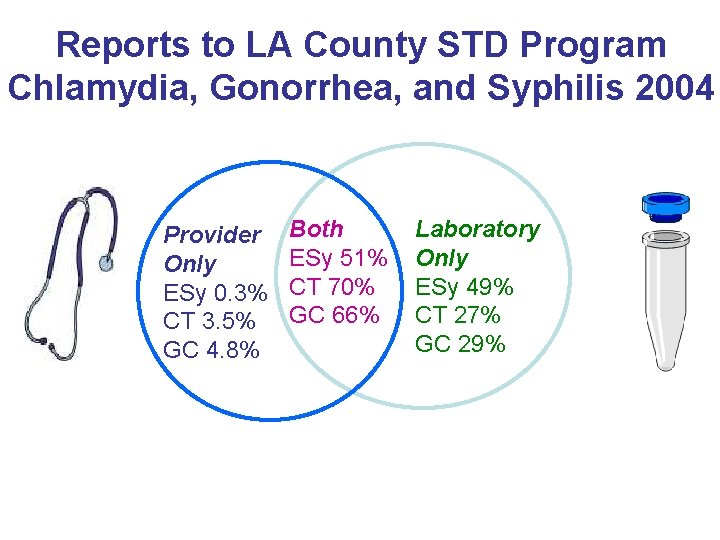
Reports to LA County STD Program Chlamydia, Gonorrhea, and Syphilis 2004 Provider Only ESy 0. 3% CT 3. 5% GC 4. 8% Both ESy 51% CT 70% GC 66% Laboratory Only ESy 49% CT 27% GC 29%

Reporting Issues • Improved enforcement of reporting laws • State investigations; non-compliance can result in loss of lab license • Identification of non-reporters is difficult • Dual reporting requirement facilitates identification • Annual laboratory survey identifies laboratories with deficient reporting practices

Reporting Issues (continued) • Heaviest burden on laboratories • Often sole reporting source • Rely on providers for patient information • Referring laboratories withhold patient / provider information from testing facilities • Increased interstate testing has accompanied growth of managed care & core laboratories • Reporting requirements vary by state

Annual Laboratory Survey • Monitor compliance with reporting guidelines • Monitor trends in test utilization & laboratory practice • Provide information on STD testing trends and recommendations • Inform laboratories about STD Program function
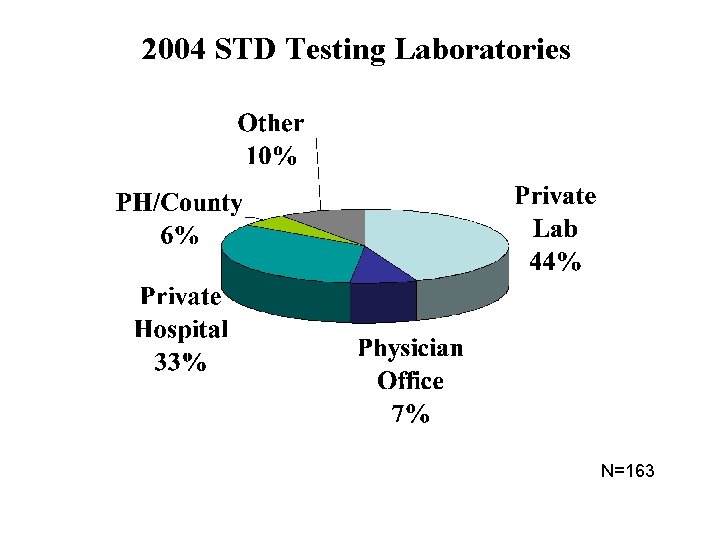
2004 STD Testing Laboratories N=163
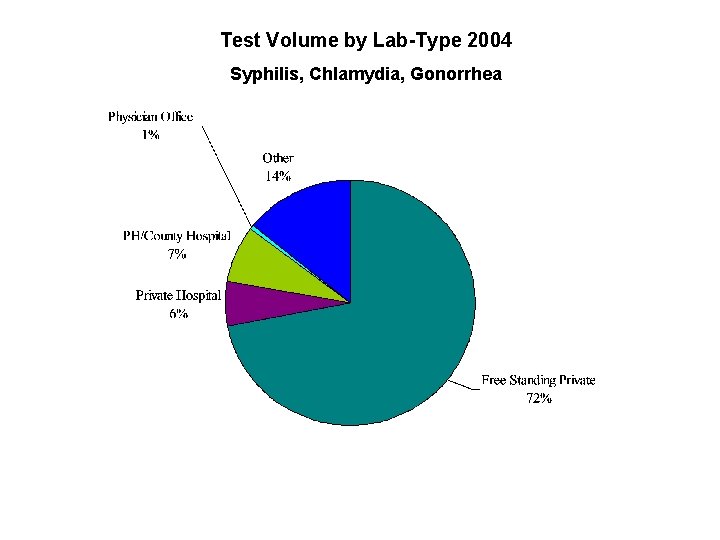
Test Volume by Lab-Type 2004 Syphilis, Chlamydia, Gonorrhea
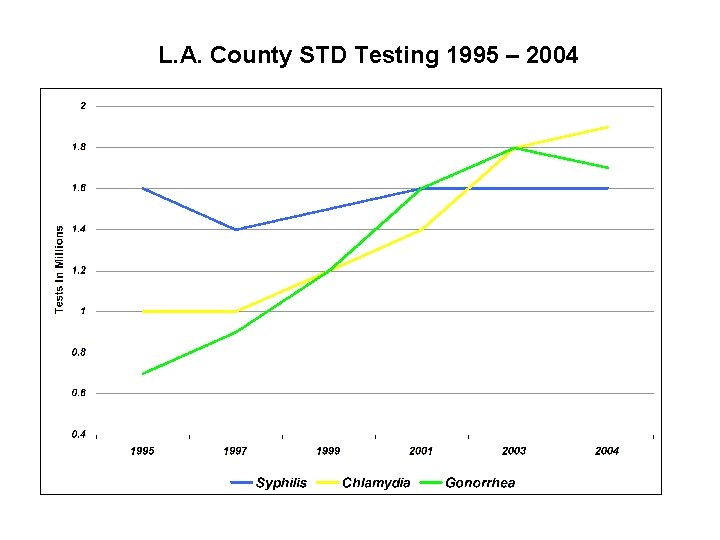
L. A. County STD Testing 1995 – 2004
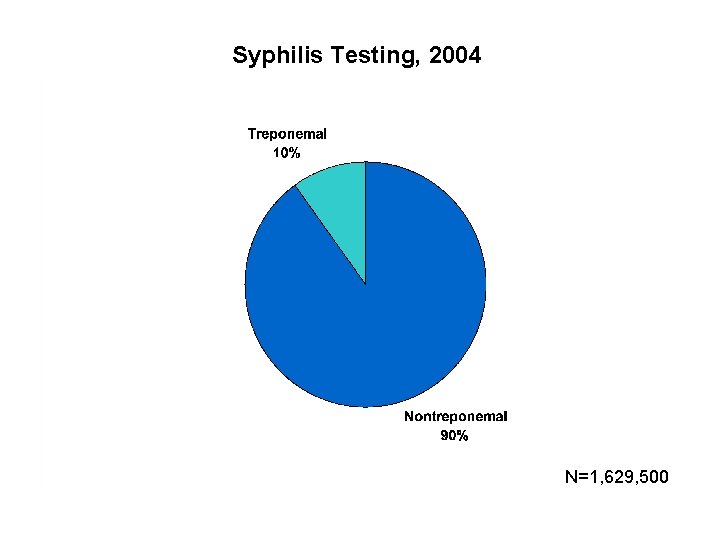
Syphilis Testing, 2004 N=1, 629, 500
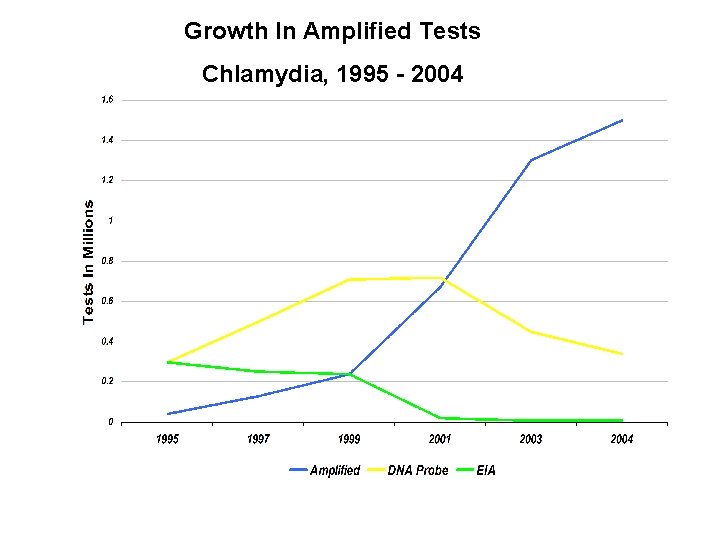
Growth In Amplified Tests Chlamydia, 1995 - 2004
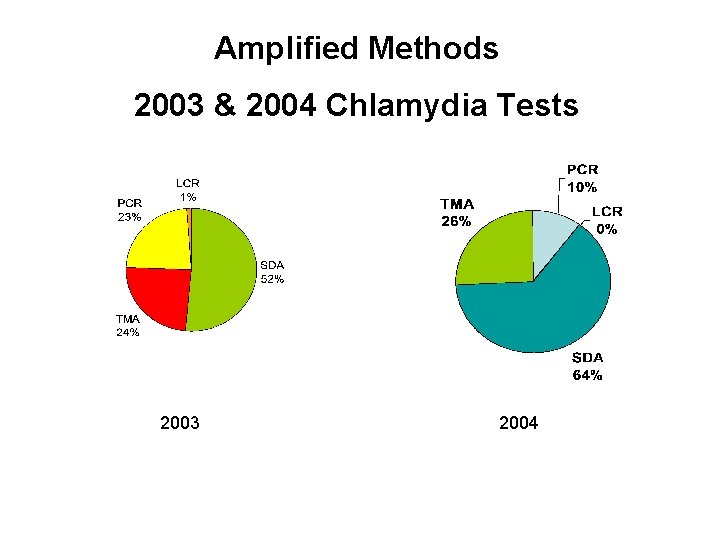
Amplified Methods 2003 & 2004 Chlamydia Tests 2003 2004
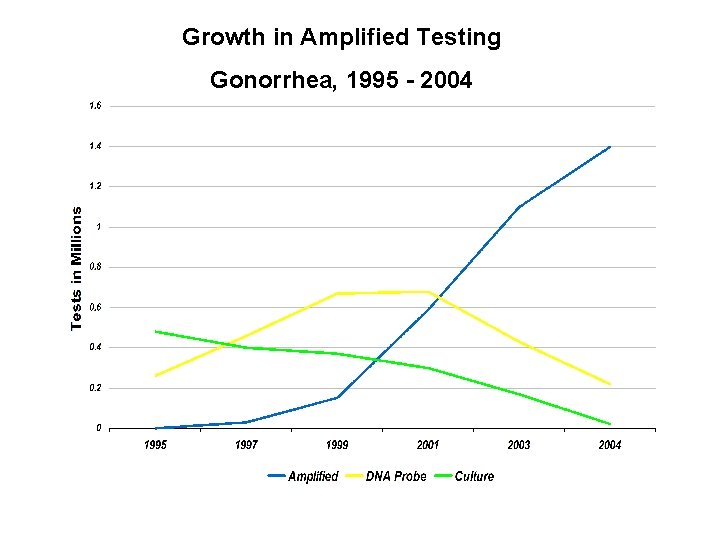
Growth in Amplified Testing Gonorrhea, 1995 - 2004
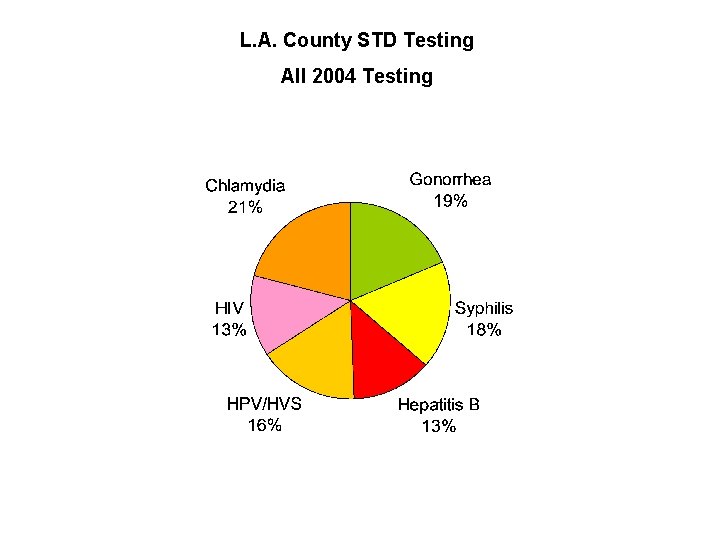
L. A. County STD Testing All 2004 Testing
- Slides: 21