Laboratory Response Capacity Coordinating Center for Infectious Diseases
Laboratory Response Capacity Coordinating Center for Infectious Diseases J. Michael Miller, Ph. D. , (D)ABMM Associate Director for Science National Center for Zoonotic, Vector-borne, and Enteric Diseases
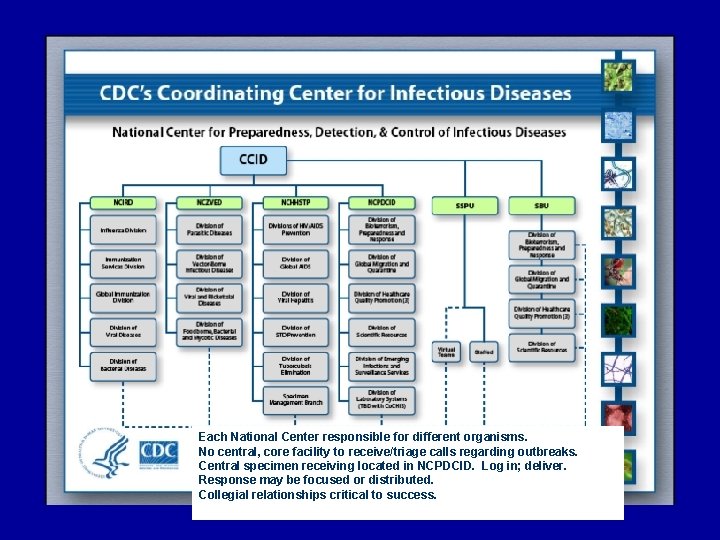
Each National Center responsible for different organisms. No central, core facility to receive/triage calls regarding outbreaks. Central specimen receiving located in NCPDCID. Log in; deliver. Response may be focused or distributed. Collegial relationships critical to success.

CCID Laboratory Capacity • Fifteen of 21 Divisions have laboratory activities • >1000 laboratories within CCID – 43 additional within NCEH – Surge capacity is robust within CCID • Estimate 40% of CCID’s ~4500 employees and contractors are laboratorians • 80 -85 CDC-Atlanta laboratories are registered for Select Agent work

Laboratory Response Activities Within CCID • • Outbreak surveillance and response Reference identification capacity Public health research and applications Pathogen discovery Vaccine discovery and adverse event tracking Repositories Preparedness and response to all public health emergencies – a hallmark of our Mission • Laboratory support – – Training (at CDC and in the field) Methods Products (test development and deployment) Service

Laboratory Partners • • Primary clients: State Public Health Agencies World Health agencies Other Federal/State agencies Private sector; non-government organizations Academia Medical community Others with public health mission

Previous Outbreaks of Unknown Etiology • Respiratory disease in attendees of an American Legion convention in Philadelphia in 1976 • Early ’ 80 s, acquired immunodeficiency syndrome initially emerges in men who have sex with men • Severe acute respiratory syndrome in 2002 -2003 • Severe pulmonary disease in the four-corners region of the U. S. • Febrile illness; rash illness; encephalitis illness; jaundice, infectious diseases from transplants; unexplained deaths Many of these were caused by novel etiologic agents that are now fully characterized

Challenges of Outbreaks of Unknown Etiology • • • 25% to 50% of cases of acute respiratory illness 50%-75% of cases of encephalitis 25% to 50% of cases of acute gastroenteritis Brainerd diarrhea Noncultivable pathogens Crohn’s disease Jaundice Non-A-E viral hepatitis Isolates that resist identification or characterization Unexplained deaths

Laboratory Tools Used in CCID Culture Cell culture Phenotypic analysis Serology Immuno-capture Western Blot Immunohistochemical analysis Histopathology Electron microscopy Mass spectroscopy PCR assays for specific pathogens Panviral PCR detection Real time PCR assays Random primer amplification DNA sequencing/analysis Parallel tag Sequencing Microarrays “Fingerprinting” technologies Generic enzyme function Target enrichment from specimens Proteomics Primer Extension Enrichment Reaction (PEER) Serum probe of translated-subtracted RNA
Examples of Surveillance Tools for Foodborne Outbreaks • Food. Net (Foodborne Diseases Active Surveillance Network) – Conducts population-based, active surveillance for laboratory-confirmed infections caused by 9 pathogens commonly transmitted through food – Campylobacter spp. , Listeria monocytogenes, Salmonella spp. , Shigella spp. , STEC O 157, Vibrio spp. , Yersinia enterocolitica, Cryptosporidium spp. , Cyclospora spp. (National Antimicrobial Resistance Monitoring System) – Conducts integrated surveillance to monitor the development of antimicrobial drug resistance among enteric pathogens from humans (CDC), foods (FDA), and animals (USDA) • NARMS • Pulse. Net USA – Standardized molecular typing of foodborne disease-causing bacteria by pulsed-field gel electrophoresis (PFGE) • Outbreak. Net – Network of state and federal public health officials who investigate foodborne disease outbreaks; Epidemiologists, microbiologists, regulatory officials in 50 states; Long-standing collaboration formalized • NEDSS (National Electronic Diseases Surveillance System) – Effort to standardize and streamline surveillance for individual cases of nationally reportable diseases – Struggling, not yet successful • From e. FORS to Outbreak Reporting System (ORS) – Will incorporate foodborne, waterborne and other enteric outbreak reporting (including norovirus) into one system – enhance user interface All require complex IT interactions but are not interconnected: data entry, tracking, reporting, access

Challenges We Face in Laboratory Response • Resolving outbreaks of unknown etiology • GMP development of assays in a “non-manufacture” arena • FDA approval of public health assays (surveillance vs diagnostic) within a timely manner – especially emergency items • Competing resources results in reduction of laboratory capacity and response • Recruitment and retention of top laboratory scientists • Developing the next generation of public health leaders • Complexity of electronic communication needs • Integrating critical public health information and tracking in a secure, efficient, and accessible format.
- Slides: 10