Laboratory Medicine Practice Guidelines LMPG Evidence Based Practice

Laboratory Medicine Practice Guidelines (LMPG) Evidence Based Practice for Point of Care Testing

National Academy of Clinical Biochemistry (NACB) Mission • The NACB is dedicated to advancing the science and practice of clinical laboratory medicine through research, education, and professional development. • NACB publishes Laboratory Medicine Practice Guidelines (LMPGs) for the application of clinical biochemistry to medical diagnosis and therapy.
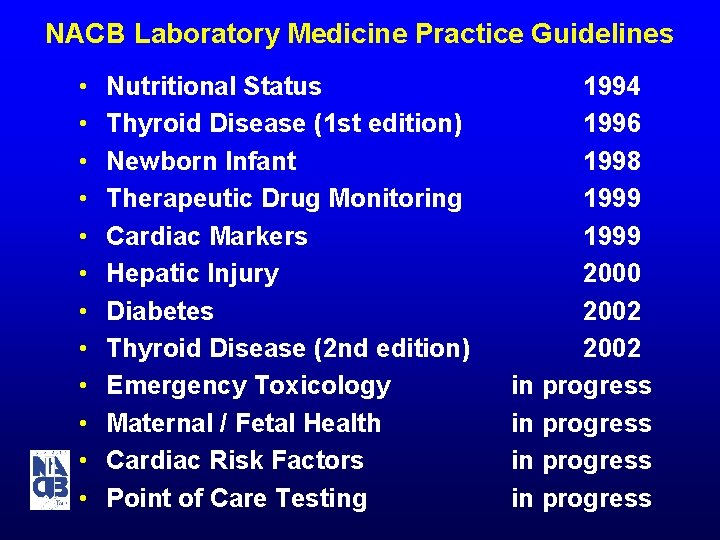
NACB Laboratory Medicine Practice Guidelines • • • Nutritional Status Thyroid Disease (1 st edition) Newborn Infant Therapeutic Drug Monitoring Cardiac Markers Hepatic Injury Diabetes Thyroid Disease (2 nd edition) Emergency Toxicology Maternal / Fetal Health Cardiac Risk Factors Point of Care Testing 1994 1996 1998 1999 2000 2002 in progress

Evidence Based Practice for POCT • POCT is an increasingly popular means of delivering laboratory testing. • When used appropriately, POCT can improve patient outcome by providing a faster result and therapeutic intervention. • However, when overutilized or incorrectly performed, POCT presents a patient risk and potential for increased cost of healthcare.

Evidence Based Practice for POCT • Clinicians, staff and laboratorians need guidance to apply POCT in the most effective manner for patient benefit. • This guidance should be based on a concurrence of the scientific evidence to date. • This LMPG will systematically review the existing evidence relating POCT to patient outcome, grade the literature, and make recommendations regarding the optimal utilization of POCT devices in patient care. • Develop liaisons with appropriate professional, clinical organizations: ADA, ACOG, American College of Cardiology, etc.

Evidence Based Practice for POCT Organizing Committee • • • James H. Nichols, Ph. D. (Chair) Naomi Aronson, Ph. D. Robert H. Christenson, Ph. D. Ellis Jacobs, Ph. D. Kent B. Lewandrowski, M. D. Rodney S. Markin, M. D. , Ph. D. Christopher Price, Ph. D. David B. Sacks, M. D. Salvador F. Sena, Ph. D. William E. Winter, M. D.

Evidence Based Practice for POCT Schedule • Fall 2002 - Form focus groups on specific topics composed of clinicians, laboratorians, industry and representatives of professional societies. • Spring 2003 - Conduct a systematic review of literature and make specific recommendations. • Summer 2003 – Internet availability of recommendations for public comment. • Fall 2003/Spring 2004 – Groups resolve comments. • Winter 2003/Spring 2004 – Internet publication of revised recommendations • Summer 2004 – Present final recommendations.
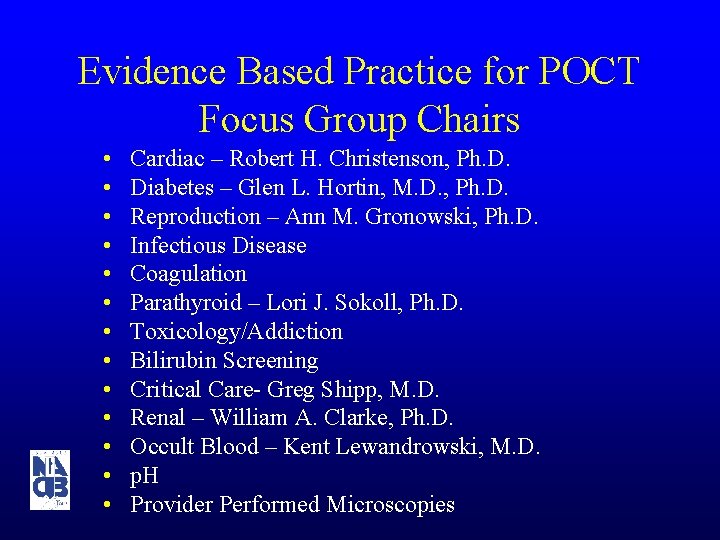
Evidence Based Practice for POCT Focus Group Chairs • • • • Cardiac – Robert H. Christenson, Ph. D. Diabetes – Glen L. Hortin, M. D. , Ph. D. Reproduction – Ann M. Gronowski, Ph. D. Infectious Disease Coagulation Parathyroid – Lori J. Sokoll, Ph. D. Toxicology/Addiction Bilirubin Screening Critical Care- Greg Shipp, M. D. Renal – William A. Clarke, Ph. D. Occult Blood – Kent Lewandrowski, M. D. p. H Provider Performed Microscopies

Evidence Based Practice for POCT • This LMPG promises to be the most comprehensive collection of our POCT knowledge base. • Recommendations from this LMPG will be useful: • To sort the facts from conjecture when implementing and utilizing POCT devices. • To establish proven applications from offlabel and alternative uses of POCT • To define the mechanisms and strategies for optimizing patient outcome.

Evidence Based Practice for POCT THE NACB WANTS YOUR INPUT

Evidence Based Practice for POCT Levels of Participation • Send POCT Concerns and Comments to Organizing Committee • Focus Group Member • Participant in Systematic Review • Develop Practice Guidelines • Review Draft Recommendations as a consumer and provide comment or suggestions.

For more information visit the NACB at www. nacb. org
- Slides: 12