Laboratory Diagnosis of selected bodyfluids Central Pathology Lab

Laboratory Diagnosis of selected bodyfluids Central Pathology Lab BMC Pleuralfluid, Asciticfluid, CSF Presenter: Mr. J. Muyombe Dept of Microbiology, Central Pathology Lab, BMC
Outline • Pleuralfluid Analysis, Asciticfluid Analysis: Sample, Tests and Interpretation • Cerebrospinalfluid: Sample, Test and Interpretation
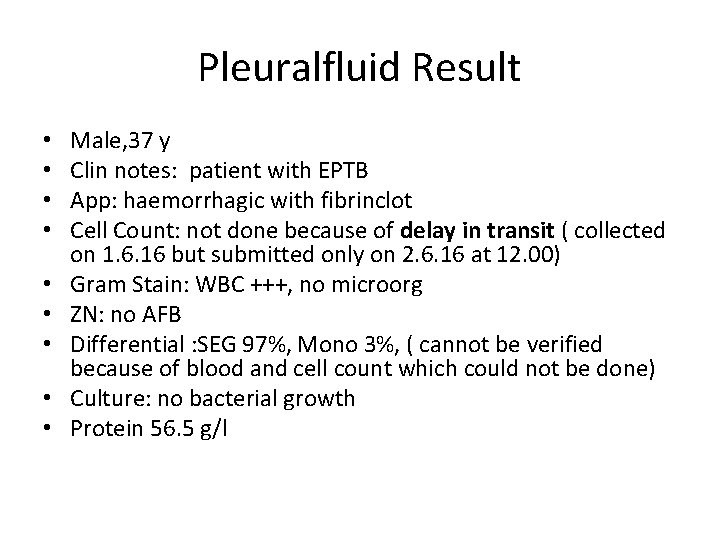
Pleuralfluid Result • • • Male, 37 y Clin notes: patient with EPTB App: haemorrhagic with fibrinclot Cell Count: not done because of delay in transit ( collected on 1. 6. 16 but submitted only on 2. 6. 16 at 12. 00) Gram Stain: WBC +++, no microorg ZN: no AFB Differential : SEG 97%, Mono 3%, ( cannot be verified because of blood and cell count which could not be done) Culture: no bacterial growth Protein 56. 5 g/l
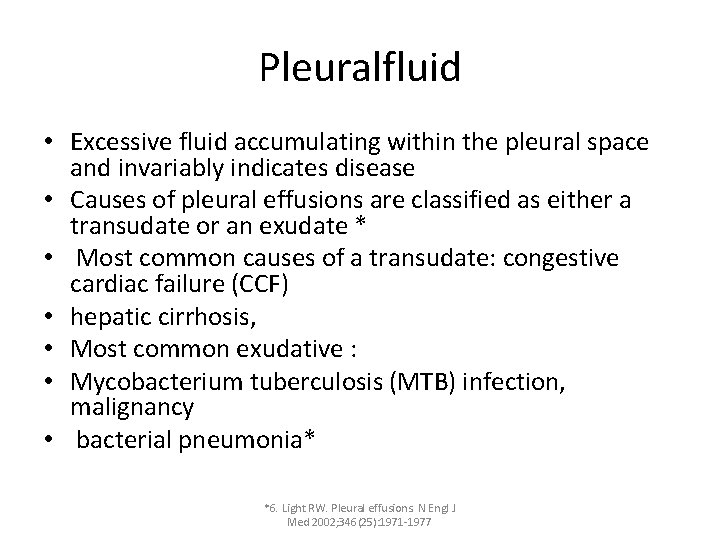
Pleuralfluid • Excessive fluid accumulating within the pleural space and invariably indicates disease • Causes of pleural effusions are classified as either a transudate or an exudate * • Most common causes of a transudate: congestive cardiac failure (CCF) • hepatic cirrhosis, • Most common exudative : • Mycobacterium tuberculosis (MTB) infection, malignancy • bacterial pneumonia* *6. Light RW. Pleural effusions. N Engl J Med 2002; 346(25): 1971 -1977

Transudate/Exudate (“traditonal”) Features Transudate Exudate Appearance Clear, strawcolured Cloudy, purulent Protein < 3 g/ dl > 3 g/dl Glucose < 3. 3 mmol/l > 3. 3 mmol/l LDH < 200 IU/l > 200 IU/l Cells < 1000/cmm > 1000/cmm
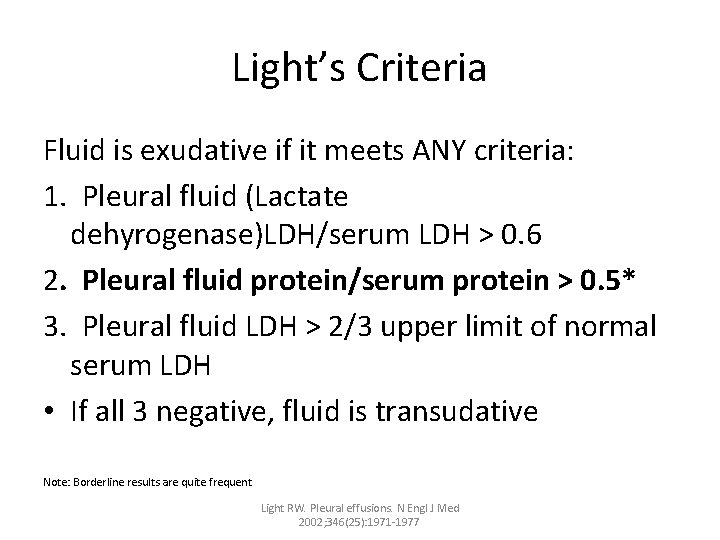
Light’s Criteria Fluid is exudative if it meets ANY criteria: 1. Pleural fluid (Lactate dehyrogenase)LDH/serum LDH > 0. 6 2. Pleural fluid protein/serum protein > 0. 5* 3. Pleural fluid LDH > 2/3 upper limit of normal serum LDH • If all 3 negative, fluid is transudative Note: Borderline results are quite frequent Light RW. Pleural effusions. N Engl J Med 2002; 346(25): 1971 -1977
Comments to Light’s Criteria • Highly sensitive for identifying an exudate • Specificity is low especially in heart failure • In case of uncertainty request for Cholesterol may help: cut-off for transsudate : 1. 6 mmol/L • But: reliability of the method for these low levels may be reduced
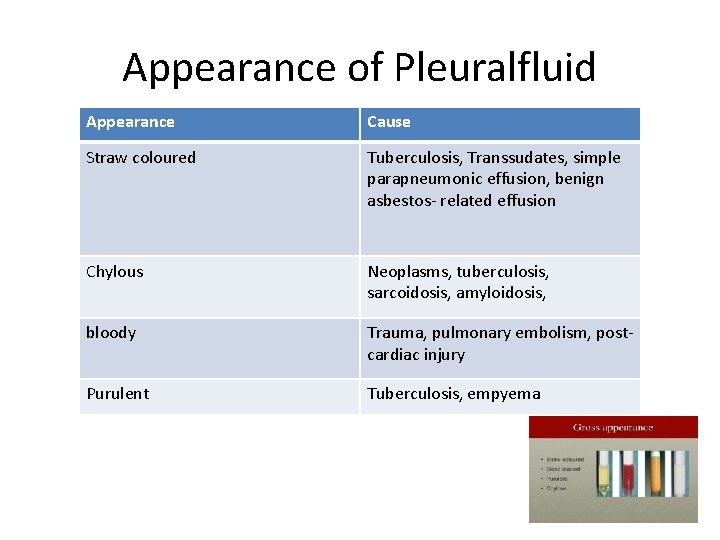
Appearance of Pleuralfluid Appearance Cause Straw coloured Tuberculosis, Transsudates, simple parapneumonic effusion, benign asbestos- related effusion Chylous Neoplasms, tuberculosis, sarcoidosis, amyloidosis, bloody Trauma, pulmonary embolism, postcardiac injury Purulent Tuberculosis, empyema

Cell Count • Transudates: few cells (less than 1000) • Exudates: Cell count may vary from 1000 to more than 50. 000/cmm.
Differential • predominantly polymorphonuclear cells: reflects an acute process; • Polymorphs are also seen in effusion caused by pulmonary embolus, tuberculosis • Lymphocytosis: 80% often the result of tuberculosis or malignancy. • However, up to 10% of tuberculosis effusions are polymorph predominant and lymphocyte-rich exudates may also be caused by sarcoidosis, rheumatoid pleuritis and chylothorax. * • If suspected malignant cells are seen: refer to Histopathologist Published Online March 14, 2005 Pleural effusion: a structured approach to care† Najib M. Rahman, Stephen J. Chapman and Robert J. O. Davies Oxford Pleural Diseases Unit, Oxford Centre for Respiratory Medicine, Churchill Hospital Oxford, UK
Microbiology • Gram Stain: positive in complicated para-pneumonic infections, empyema • AFB Smear: rarely positive even if highly suspicious for TB. WHO recommends also Gene. Xpert • Note: 48 – 96% of tuberculous smears and culture are negative * • Culture : positive as for Gram, TB culture: rarely positive * *RESPIRATORY CARE • OCTOBER 2012 VOL 57 NO 10
Other possible tests • Biomarkers like ADA ( Adenosine-de-aminase) are considered useful in the diagnosis of TB • Cost-effective in areas with high prevalence? • Specific Gravity ( cut-off 1. 015 -1. 018) • Protein (Fluid): (cut-off 2. 5 – 3. 0 g/l)
Submitting samples for analysis of Pleuralfluid • STERILE container (red-top) 4 ml • ( less preferred: plain red-top container. • Do not use red-top tubes with clotting activators) • EDTA container • Samples should be processed within 4 hours but EDTA samples can be stored up to 24 hours at 4 ° C
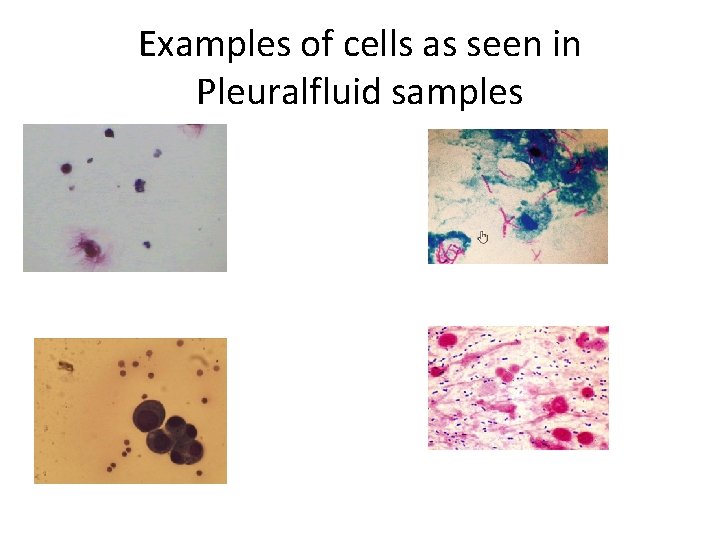
Examples of cells as seen in Pleuralfluid samples
Ascitesfluid Result • 48 y, F ( Sampled on 16. 6. 16, received in the lab on 17. 6. 16) • Clin notes: Schistosomiasis with massive ascites • App yellow, • Cell Count : 7/cmm • Prot 59. 7 g/l • Gram: Epithelialcells ++, no microorg • ZN: no AFB • Differential: cell debris only • Culture: NBG
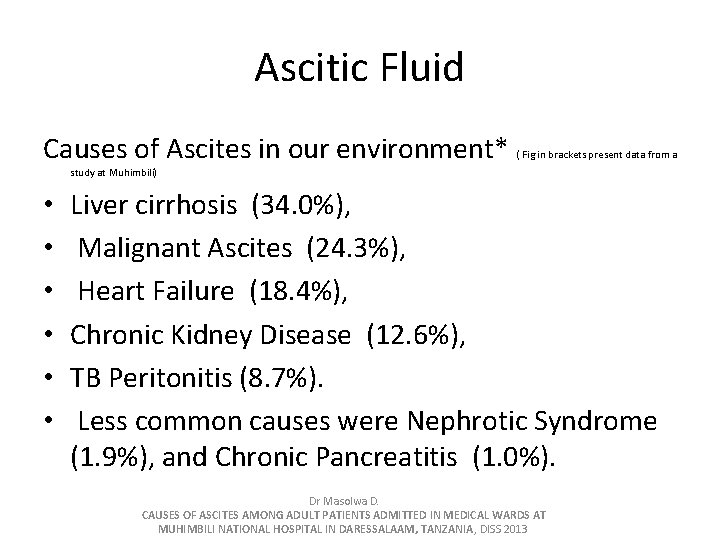
Ascitic Fluid Causes of Ascites in our environment* ( Fig in brackets present data from a study at Muhimbili) • • • Liver cirrhosis (34. 0%), Malignant Ascites (24. 3%), Heart Failure (18. 4%), Chronic Kidney Disease (12. 6%), TB Peritonitis (8. 7%). Less common causes were Nephrotic Syndrome (1. 9%), and Chronic Pancreatitis (1. 0%). Dr Masolwa D. CAUSES OF ASCITES AMONG ADULT PATIENTS ADMITTED IN MEDICAL WARDS AT MUHIMBILI NATIONAL HOSPITAL IN DARESSALAAM, TANZANIA, DISS 2013

Investigation • Transudate/Exudate • Cell count with diff • Albumin • LDH • Total protein • Glucose ( if Fluoride Oxalate Container available) • Gram stain/Culture • AFB Stain with culture • Cytology If indicated: • Fungal stain and Culture – Esp. if peritoneal dialysis catheter
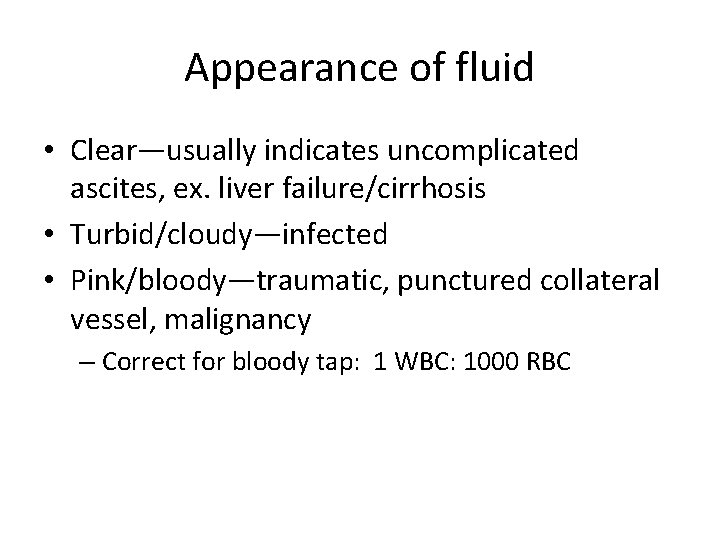
Appearance of fluid • Clear—usually indicates uncomplicated ascites, ex. liver failure/cirrhosis • Turbid/cloudy—infected • Pink/bloody—traumatic, punctured collateral vessel, malignancy – Correct for bloody tap: 1 WBC: 1000 RBC

Serum-to-ascites albumin gradient (SAAG) = Serum albumin – ascitic fluid albumin If the gradient is > 1. 1: • Portal HTN (drives fluids into peritoneum) • cirrhosis, alcoholic hepatitis, CHF, If the gradient is < 1. 1: (protein leaks into peritoneum and fluid follows) • Peritoneal carcinomatosis, peritoneal TB, pancreatitis, nephrotic syndrome, peritonitis
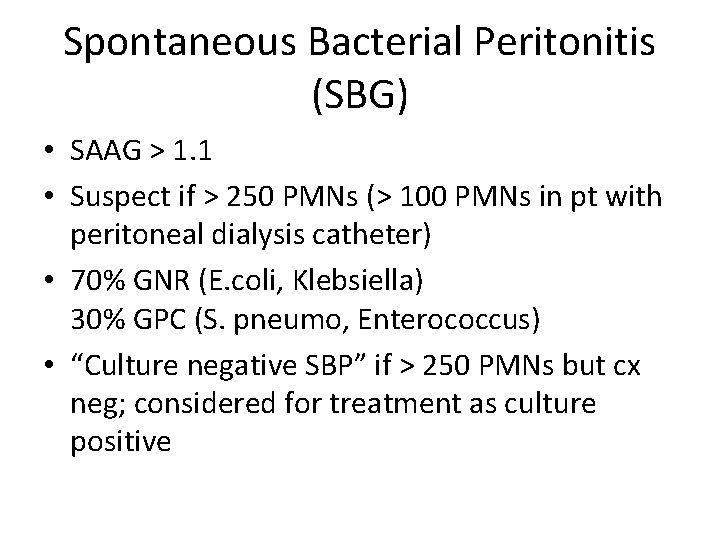
Spontaneous Bacterial Peritonitis (SBG) • SAAG > 1. 1 • Suspect if > 250 PMNs (> 100 PMNs in pt with peritoneal dialysis catheter) • 70% GNR (E. coli, Klebsiella) 30% GPC (S. pneumo, Enterococcus) • “Culture negative SBP” if > 250 PMNs but cx neg; considered for treatment as culture positive
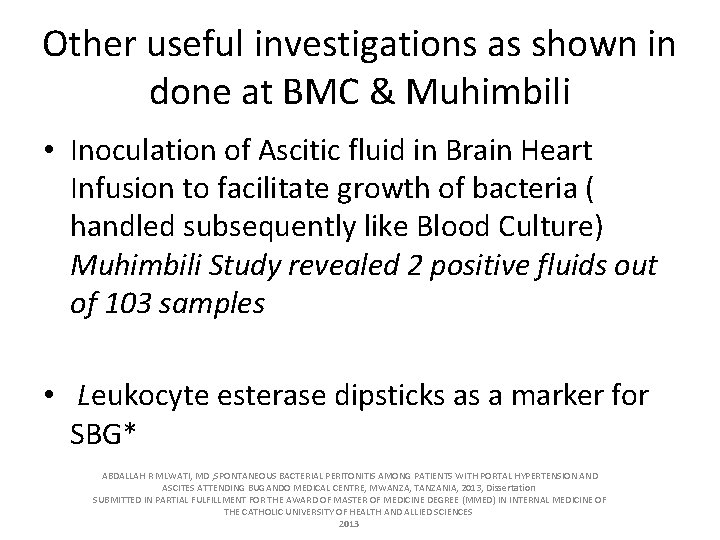
Other useful investigations as shown in done at BMC & Muhimbili • Inoculation of Ascitic fluid in Brain Heart Infusion to facilitate growth of bacteria ( handled subsequently like Blood Culture) Muhimbili Study revealed 2 positive fluids out of 103 samples • Leukocyte esterase dipsticks as a marker for SBG* ABDALLAH R MLWATI, MD , SPONTANEOUS BACTERIAL PERITONITIS AMONG PATIENTS WITH PORTAL HYPERTENSION AND ASCITES ATTENDING BUGANDO MEDICAL CENTRE, MWANZA, TANZANIA, 2013, Dissertation SUBMITTED IN PARTIAL FULFILLMENT FOR THE AWARD OF MASTER OF MEDICINE DEGREE (MMED) IN INTERNAL MEDICINE OF THE CATHOLIC UNIVERSITY OF HEALTH AND ALLIED SCIENCES 2013
Pictures of Ascitic fluid
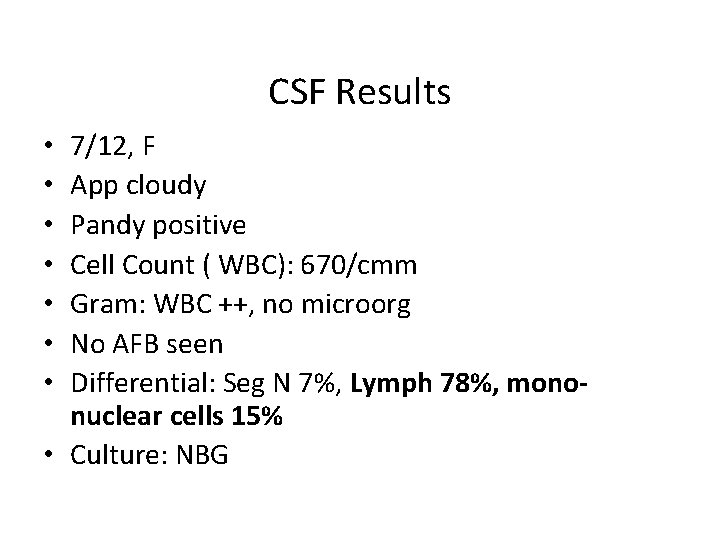
CSF Results 7/12, F App cloudy Pandy positive Cell Count ( WBC): 670/cmm Gram: WBC ++, no microorg No AFB seen Differential: Seg N 7%, Lymph 78%, mononuclear cells 15% • Culture: NBG • •
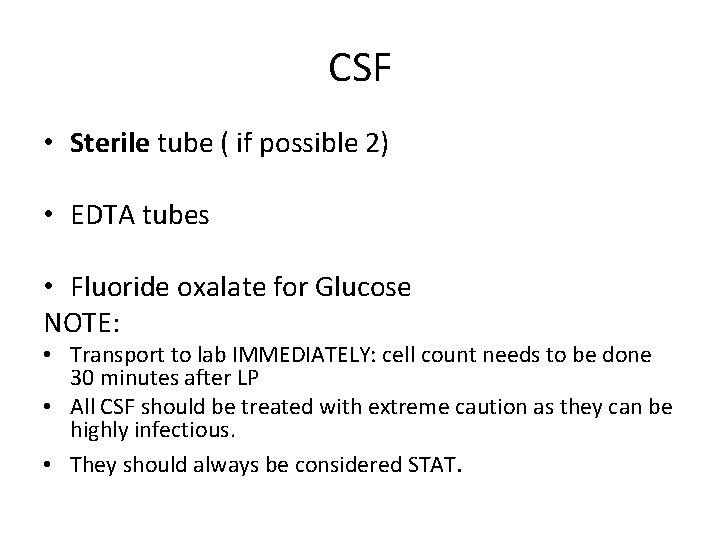
CSF • Sterile tube ( if possible 2) • EDTA tubes • Fluoride oxalate for Glucose NOTE: • Transport to lab IMMEDIATELY: cell count needs to be done 30 minutes after LP • All CSF should be treated with extreme caution as they can be highly infectious. • They should always be considered STAT.

Evaluation of CSF • Appearance • Protein ( as emergency test: Pandy) • Cell Count • India Ink, Gram, AFB, Leishman & Culture • If required: CRAG

Normal CSF Values • • Clear, colourless < 5 WBBC’s Protein 23 -38 mg/dl (alt. 14 -45) Glucose - 60% of serum level (75 -100) • Pandy Test: normal
Appearance • Descriptive terms used: clear, hazy, cloudy, turbid, bloody, xanthochromic. • Quantification if indicated: slight, moderate, marked, or grossly. xanthochromia — term used only for CSF to describe a yellowing discoloration of the supernatant. • Indicates presence of old blood: RBCs have been present in the CSF for an extended period of time, have broken down, released their hemoglobin, and the Hgb has been converted to bilirubin • Elevated serum bilirubin • Increased Protein

Haemorrhagic CSF • After centrifugation: • If supernatant clear and colourless with deposit of fresh RBC: traumatic LP • If supernatant haemolytic or xanthochromic: degraded RBCs
Cell Count • Undiluted CSF • No electronic counting • If haemorrhagic CSF: Total Cell. Count and WBC • ( Interpretation: 1000 RBC increase WBC by 1 cell)

Why Cell Count within 30 minutes? • Cells can already lyse within the first hour, which can decrease or falsify the value of the cell count. • The shorter lifespan of the cells in the cerebrospinal fluid, especially that of the granulocytes, is attributable to the significantly lower protein content in the cerebrospinal fluid • Exposure to light or contact with oxygen can shorten the in vitro half life of granulocytes even more • Changes in p. H when exposed to CO 2 ( alkaline p. H contributes to cell degradation)

Gram Stain • Neonates: E Coli or Group B Strep • Children > 6 mo. and young adults: Streptococcus pneumoniae, Haemophilus influenza or Neisseria meningitidis • Adults: Neisseria meningitidis or Streptococcus pneumoniae • Staph aureus if a shunt is present • Immunocompromised patient: Cryptococcus neoformans, Candida albicans, Coccidiodes, or any opportunistic organism
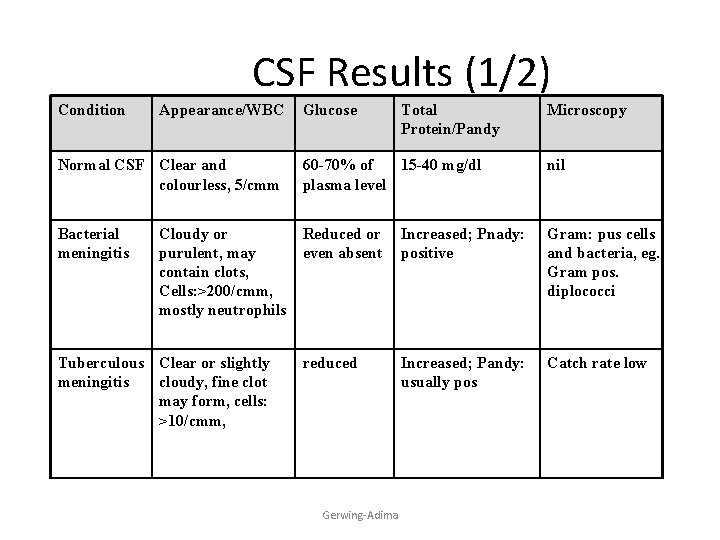
CSF Results (1/2) Condition Appearance/WBC Glucose Total Protein/Pandy Microscopy Normal CSF Clear and colourless, 5/cmm 60 -70% of 15 -40 mg/dl plasma level nil Bacterial meningitis Reduced or even absent Increased; Pnady: positive Gram: pus cells and bacteria, eg. Gram pos. diplococci reduced Increased; Pandy: usually pos Catch rate low Cloudy or purulent, may contain clots, Cells: >200/cmm, mostly neutrophils Tuberculous Clear or slightly meningitis cloudy, fine clot may form, cells: >10/cmm, Gerwing-Adima
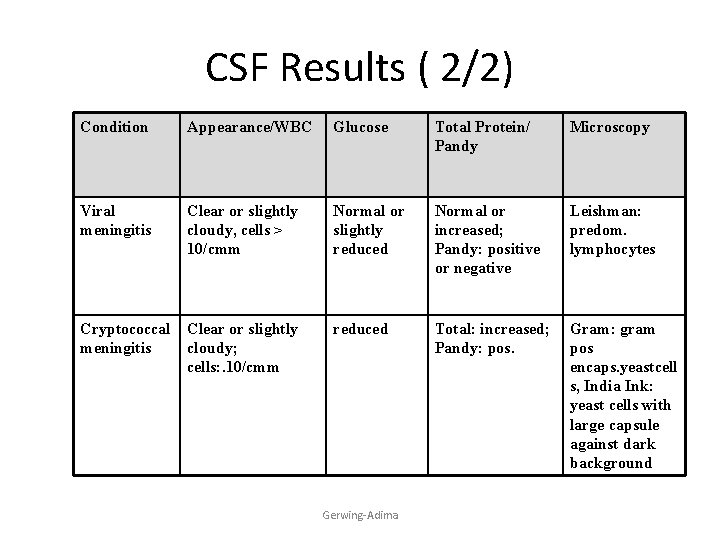
CSF Results ( 2/2) Condition Appearance/WBC Glucose Total Protein/ Pandy Microscopy Viral meningitis Clear or slightly cloudy, cells > 10/cmm Normal or slightly reduced Normal or increased; Pandy: positive or negative Leishman: predom. lymphocytes Cryptococcal meningitis Clear or slightly cloudy; cells: . 10/cmm reduced Total: increased; Pandy: pos. Gram: gram pos encaps. yeastcell s, India Ink: yeast cells with large capsule against dark background Gerwing-Adima
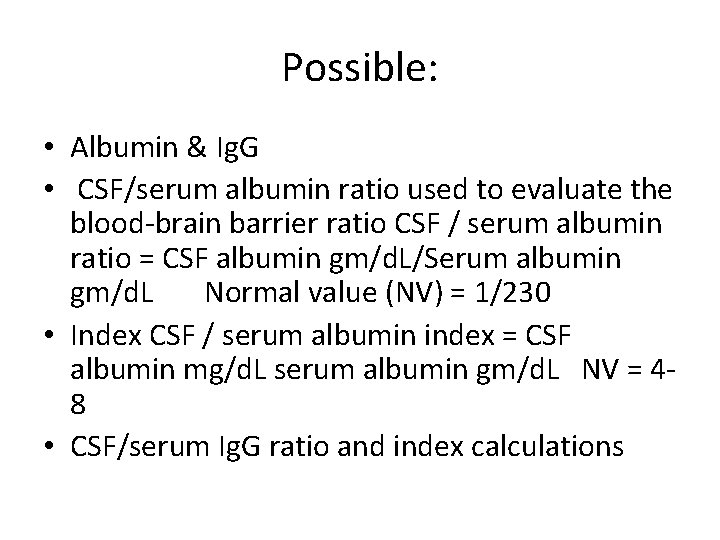
Possible: • Albumin & Ig. G • CSF/serum albumin ratio used to evaluate the blood-brain barrier ratio CSF / serum albumin ratio = CSF albumin gm/d. L/Serum albumin gm/d. L Normal value (NV) = 1/230 • Index CSF / serum albumin index = CSF albumin mg/d. L serum albumin gm/d. L NV = 48 • CSF/serum Ig. G ratio and index calculations
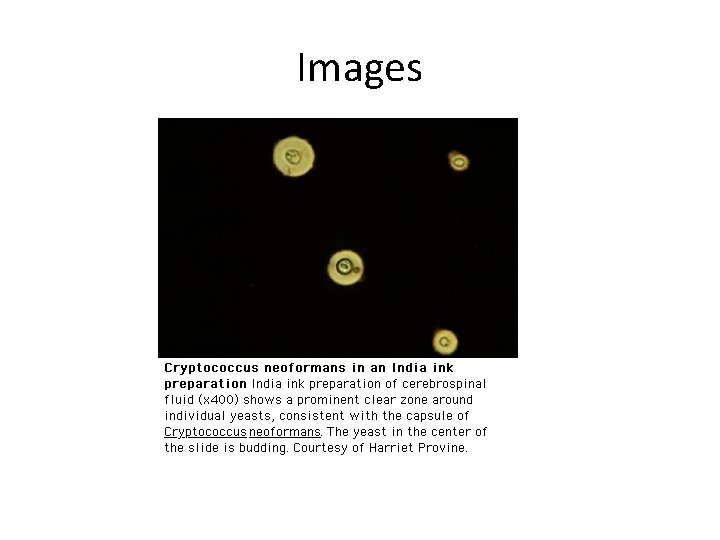
Images
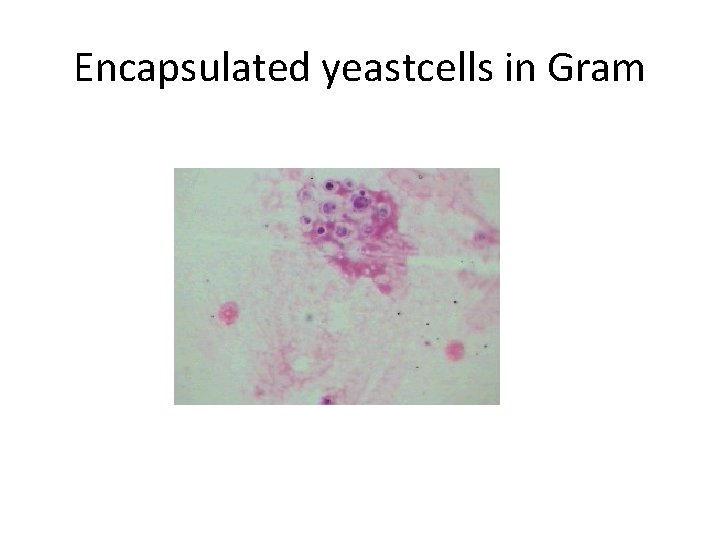
Encapsulated yeastcells in Gram
Mono-nuclear cells in CSF
- Slides: 37