Laboratory Diagnosis in Thalassemia and Hemoglobinopathies Ahmad Shihada


Laboratory Diagnosis in Thalassemia and Hemoglobinopathies Ahmad Shihada Silmi Msc, FIBMS Staff Specialist in Hematology Medical Technology Department Islamic University of Gaza
Thalassemia and hemoglobinopathies n n Disorders of globin chain production as a consequences of globin gene defects As a results, hemoglobin productions are also affected. Also, some properties of red blood cells are also affected. Thus it can be recognized by various hematological laboratory tests. 3

Making diagnosis of thalassemia n n n History retrieve Physical examination Laboratory investigations 4
Laboratory Thalassemia Diagnosis n n n Red Cell Studies : CBC, One- Tube OF Test, DCIP Test Hb Studies : Electrophoresis, Microcolumn chromatography, Alkali Denaturation Test, HPLC/LPLC, Imnunologic Detection, Acid elution test DNA studies : Gene mapping, PCR, Nt sequencing, RFLP analysis 5

Blood sample *Fresh venous blood sample stored in EDTA (3 -5 ml) is enough. *This blood sample is used for both RBC studies, Hb studies and DNA studies. 6

RBC studies 7
CBC n n CBC (automate is a must) for red blood parameters including Hb, Hct, RBC indicies and RBC morphology examination (already trained) Hb H inclusion body test 8
Hb and Hct n Low in thalassemia disease n Normal or slightly low in heterozygote – Hb < 6 g% in thal major – Hb 6 -10 g% in thal intermedia – Hb 10 -12 g% in thal minor or thal trait – ( Male ~15 g%, Female ~ 13 g%) 9

MCV, MCH n n n Cut-off level : MCV 80 fl, MCH 20 pg Low in thalassemia diseases and thalassemia trait Normal or slightly low in a-thal-2 trait, Hb. E trait, Hb CS trait 10

RBC Morphology n n Thalassemia disease : Hypochromia, Anisocytosis, Poikilocytosis, Polychromasia, Target cells, Basophilic Stippling, NRBC Thalassemia Trait and Homo E : Modest change in RBC morphology 11
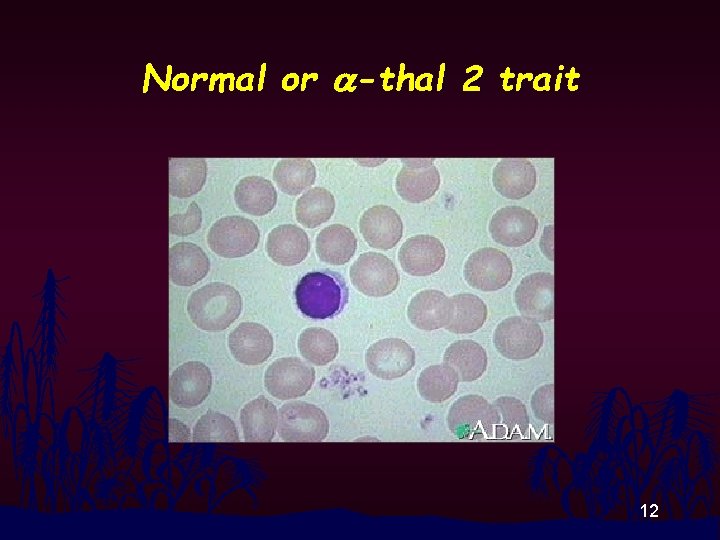
Normal or a-thal 2 trait 12
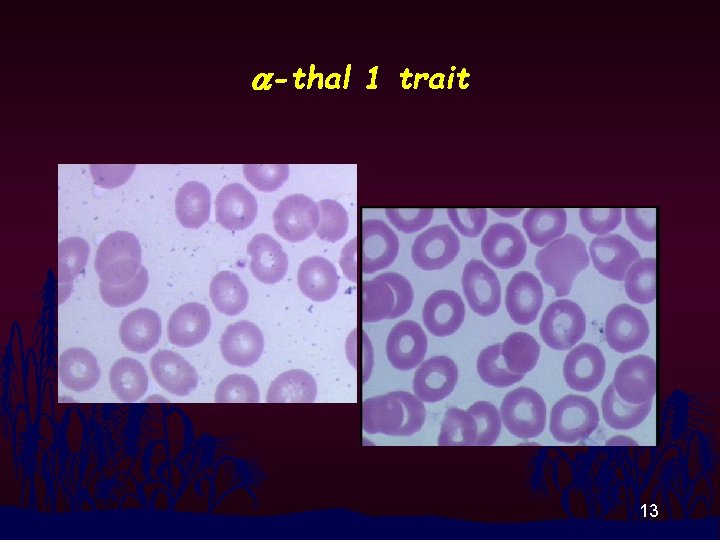
a-thal 1 trait 13
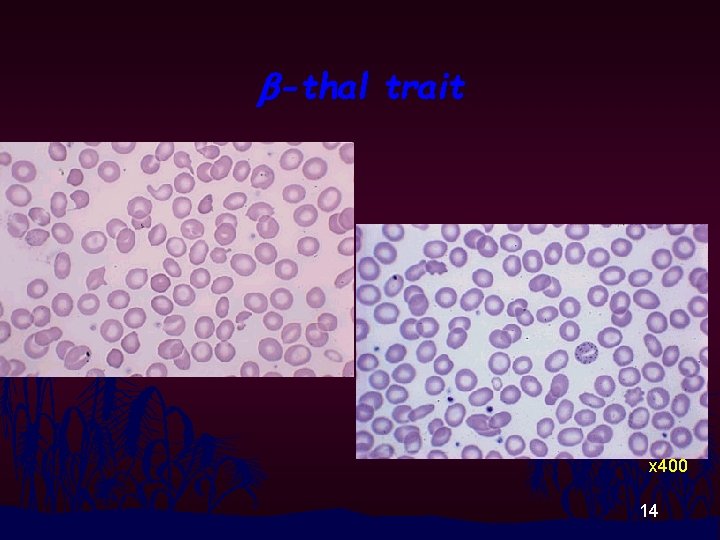
b-thal trait x 400 14
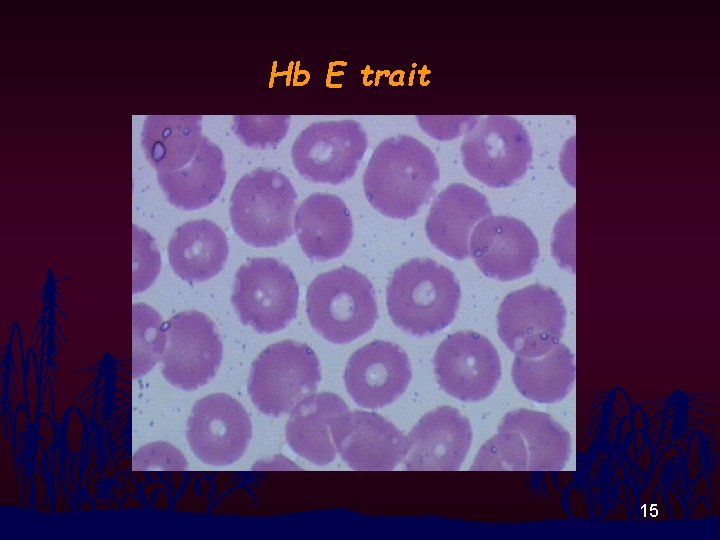
Hb E trait 15
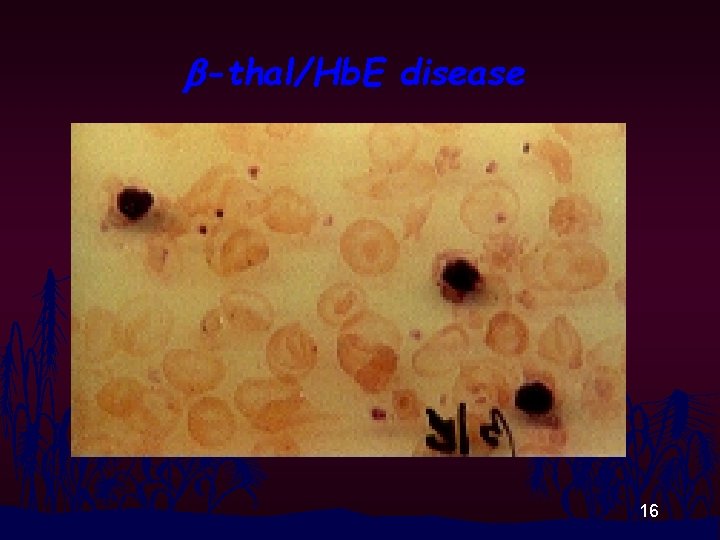
b-thal/Hb. E disease 16
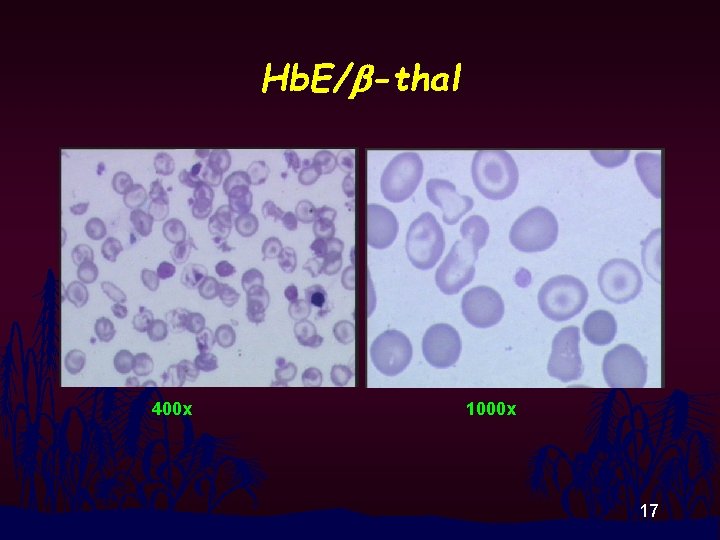
Hb. E/b-thal 400 x 1000 x 17
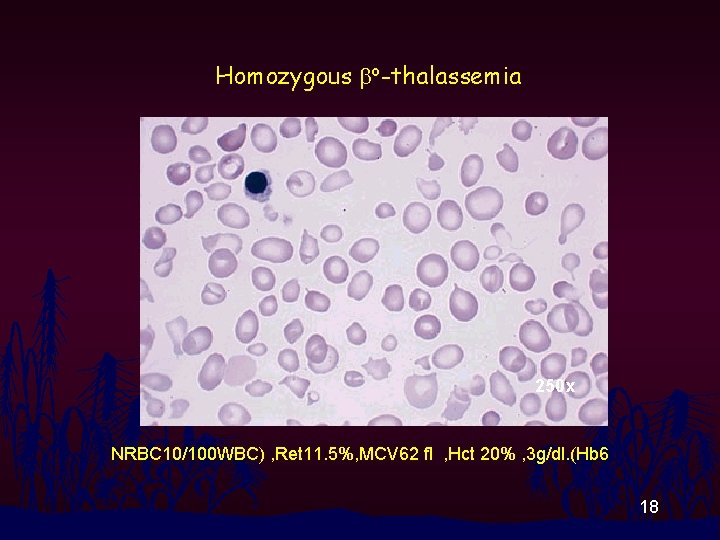
Homozygous bo-thalassemia 250 x NRBC 10/100 WBC) , Ret 11. 5%, MCV 62 fl , Hct 20% , 3 g/dl. (Hb 6 18
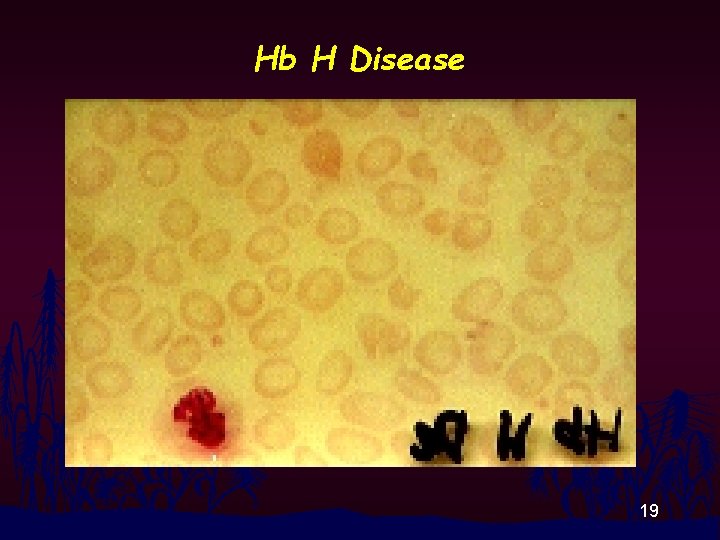
Hb H Disease 19

Hb Bart’s Hydrops Fetalis 20
Hb H inclusion body test Principle n Hb H (b 4) is an unstable hemoglobin commonly seen in a-thalassemia. On incubation with some oxidative chemicals such as brilliant cresyl blue (BCB), Hb. H is oxidised, denatured and precipitated in the erythrocytes and seen as small, evenlydistributed, intra-erythrocytic blue dots which termed Hb. H inclusion bodies. 21

Hb H inclusion body test Reagents : n As for reticulocyte count Step-by-step procedure : The stain and incubation are as for reticulocyte count But look for red blood cells containing Hb. H inclusion bodies and report as numbers of those red blood cells in certain amount of total red blood cells examined. If numerous Hb. H inclusion body containing eryhthrocytes are seen : Report in % If few or rare Hb. H inclusion body containing eryhthrocytes are seen : Report in actual numbers of those rbc/30, 000 rbc 22
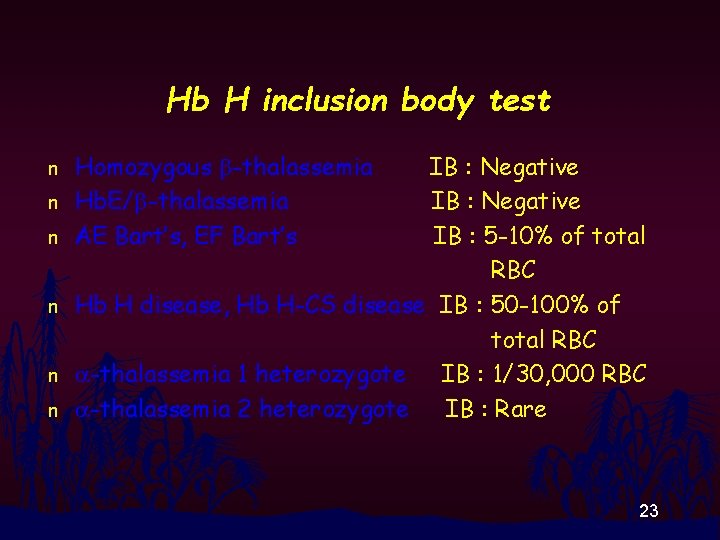
Hb H inclusion body test n n n Homozygous b-thalassemia Hb. E/b-thalassemia AE Bart’s, EF Bart’s IB : Negative IB : 5 -10% of total RBC Hb H disease, Hb H-CS disease IB : 50 -100% of total RBC a-thalassemia 1 heterozygote IB : 1/30, 000 RBC a-thalassemia 2 heterozygote IB : Rare 23
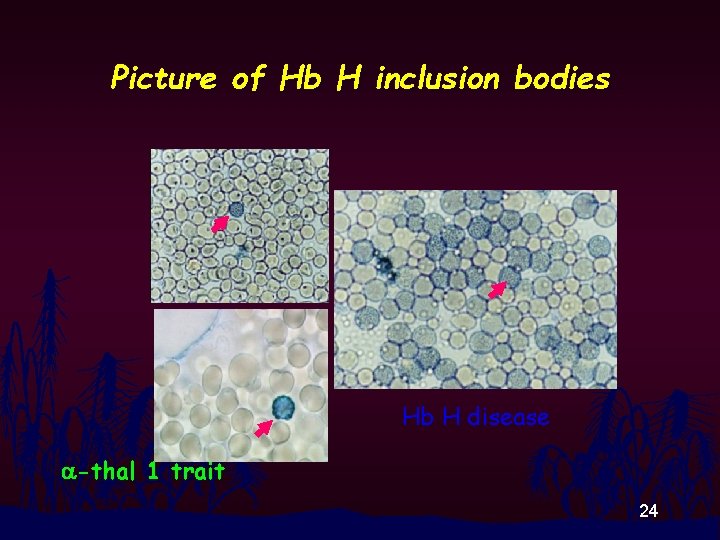
Picture of Hb H inclusion bodies Hb H disease a-thal 1 trait 24

One-tube OF test Principle n At a constant hypotonic Na. Cl solution of 0. 36% (w/v), hypochromic red blood cells are able to uphold certain amount of water and remain intact whereas the normal erythrocytes cannot and explode. 25

One-tube OF test Reagent : 0. 36% Buffered Saline Solution (BSS) Procedure n Mix 20 ul EDTA blood with 5 ml 0. 36% BSS n Stand at RT for 5 min. n Visualize for hemolysis -If clear red solution is observed : negative -If turbid red solution is observed : positive n 26

One-tube OF test (0. 36 %BSS( Negative Positive 27

One Tube OF Test Thalassemia diseases b-thal trait a-thal-1 trait a-thal-2 trait-/+ Hb. E trait-/+ Homo E + + + -/+ 28

DCIP precipitation test Principle n Hb. E (a 2 b. E 2) has loose contact between a 1 and b 1 -globin chains. When it is incubated with dichlorophenol indophenol (DCIP); oxidizing agent, it will be denatured and precipitated. The reaction is stopped by adding ascorbic acid and the denatured Hb. E precipitates. 29

DCIP test Reagent : DCIP reagent Procedure n Mix 20 ul packed red cell with 5 ml DCIP reagent in 13 x 100 test tube n Incubate the mixture at 37 C water bath for 60 min. n Look for precipitation before or after addition of 5% ascorbic acid n Report n 30

DCIP test Ways to report n Negative : No precipitation n Positive : Precipitation seen 31

DCIP test After adding 5% ascorbic acid Pos Neg Before adding 5% ascorbic acid Blk Neg Pos 32

DCIP Test Normal Homo E Hb. E trait b-thal/Hb. E disease Hb. H disease Negative 3+-4+ 1+-2+ Report as positive or negative 33
Hb studies 34

Hemolysate preparation • Centrifuge EDTA blood at 3000 -5000 rpm and remove plasma • Wash packed red cell with NSS for three time and remove supernatant as much as possible at the last washing round • Add DW 1. 5 time the volume of PRC and mix vigorously • Add CCl 4 to the half of the volume of lysed red cells and mix vigorously • Centrifuge 3000 -5000 rpm and collect the upper red portion which is “Hemolysate or Hemoglobin solution) 35
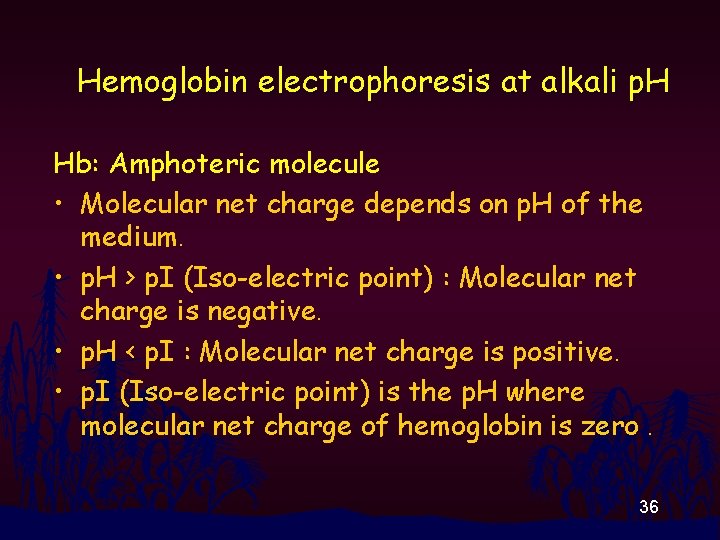
Hemoglobin electrophoresis at alkali p. H Hb: Amphoteric molecule • Molecular net charge depends on p. H of the medium. • p. H > p. I (Iso-electric point) : Molecular net charge is negative. • p. H < p. I : Molecular net charge is positive. • p. I (Iso-electric point) is the p. H where molecular net charge of hemoglobin is zero. 36
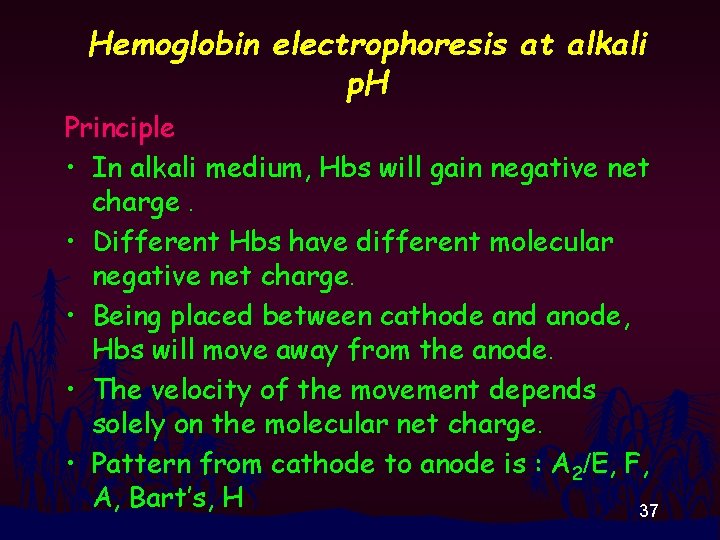
Hemoglobin electrophoresis at alkali p. H Principle • In alkali medium, Hbs will gain negative net charge. • Different Hbs have different molecular negative net charge. • Being placed between cathode and anode, Hbs will move away from the anode. • The velocity of the movement depends solely on the molecular net charge. • Pattern from cathode to anode is : A 2/E, F, A, Bart’s, H 37

Hemoglobin electrophoresis at alkali p. H Reagent : Tris-EDTABorate (TBE) p. H 8. 4 -8. 6 38

Equipment • • • . 1 Power supply for 500 V. 2 Electrophoretic chamber. 3 Cellulose acetate plate. 4 Sample applicator. 5 Stain box. 6 Large filter paper or blotter 39
Equipment Sample applicator Sample preparation well Aligning base 40
Equipment Cellulose acetate plate Blotter 41

Equipment Power supply Cellulose acetate plate Electrophoretic chamber http: //sun. science. wayne. edu/~hhmi/gifs/elec 1. jpg 42

Equipment 43

Specimens • Hb in the solution or“ hemolysate. ” 44

Procedure • Hemolysate in wells • Serum applicator dipped and applied on soaked cellulose acetate plate • Place cellulose acetate, face-down, in electrophoretic chamber. • Run elctophoresis at 3 00 volts for 10 -2 0 min. • Stained with Ponceau S 45

Ponceau S staining n n n Dip cellulose acetate plate in the stain and leave for 5 min Wash with destaining solution (5% HOAc) twice and 5 min each time or until background becomes white Read Hb bands 46
During electrophoresis 47

Ponceau S and Destaining solution 48
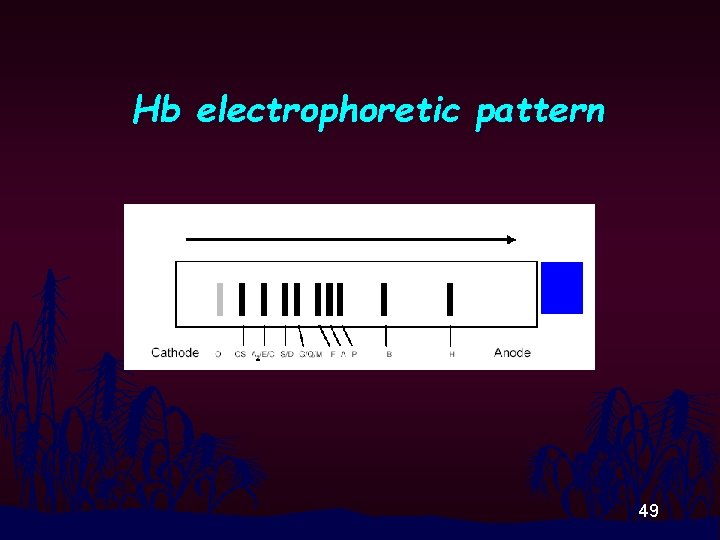
Hb electrophoretic pattern 49
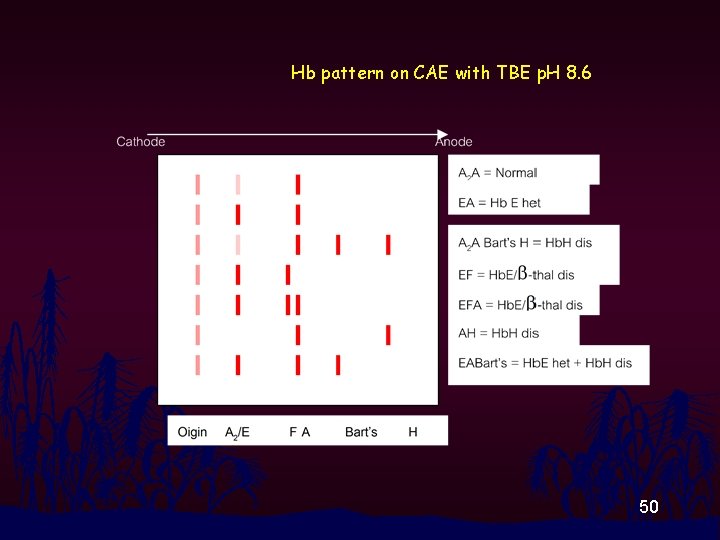
Hb pattern on CAE with TBE p. H 8. 6 50
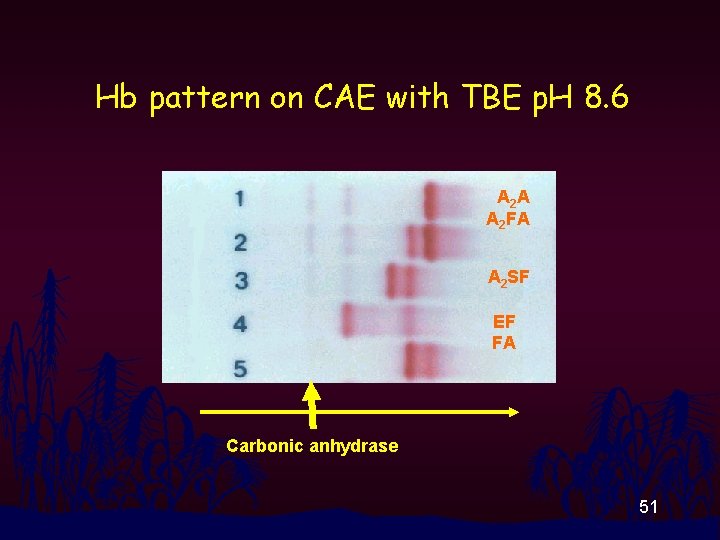
Hb pattern on CAE with TBE p. H 8. 6 A 2 A A 2 FA A 2 SF EF FA Carbonic anhydrase 51
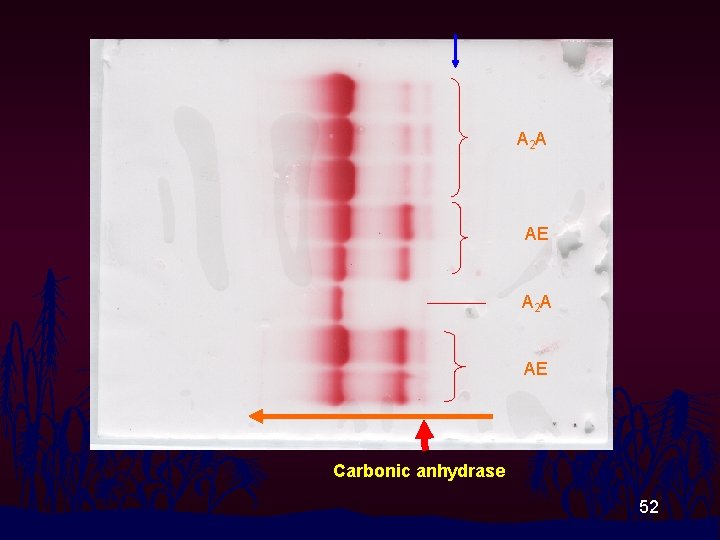
A 2 A AE Carbonic anhydrase 52
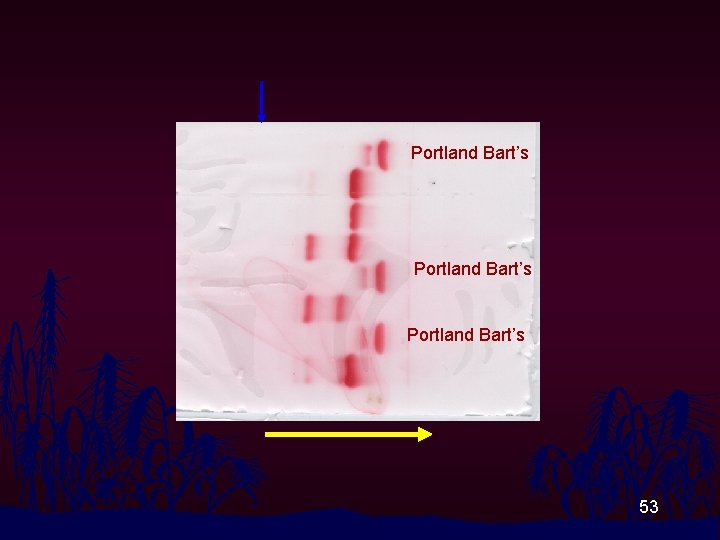
Portland Bart’s 53
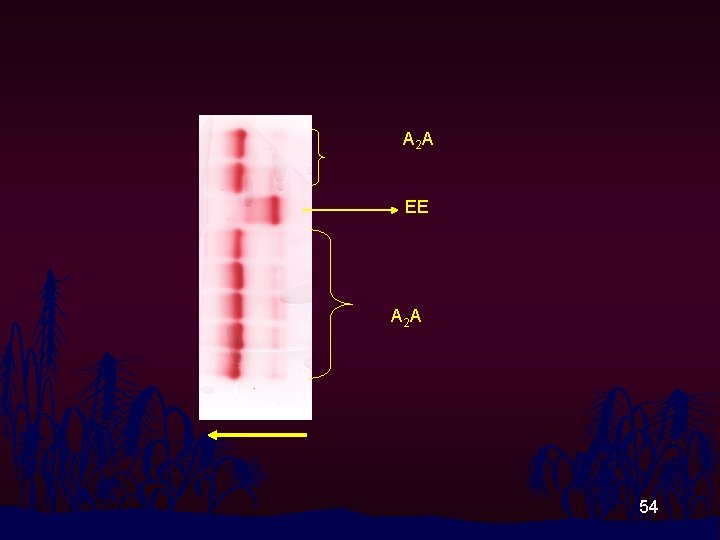
A 2 A EE A 2 A 54

Microcolumn chromatography Principle • In basic (alkali) solution, net charge of Hbs becomes negative. • Different Hbs have different negative net charge. • On passing through a colume packed with DEAE-cellulose or sephadex resin, different Hbs bind resin at different strength. 55

Microcolumn chromatography Principle • On passing Cl- ion through column, it will elute Hb off the resin. • Order of Hbs being eluted is dependent on negative net charge. 56
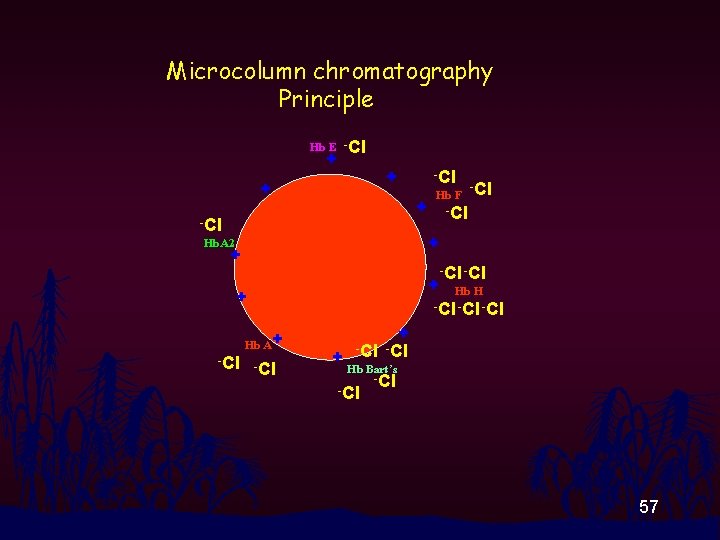
Microcolumn chromatography Principle Hb E -Cl + + + -Cl Hb F -Cl + -Cl Hb. A 2 + + -Cl + Hb H -Cl -Cl + Hb A+ -Cl + + -Cl Hb Bart’s -Cl 57

Microcolumn chromatography Procedure n Fill pre-swollened DEAE-Sephadex A-50 into Pastuer pipette n Dilute 200 ul hemolysate with 20 ml THK buffer p. H 8. 5 n Apply 10 ml diluted hemolysate into the column, overlayer column with the resin n Equilibrate with 5 ml THK p. H 8. 5 n Move column to new tube and elute with 30 ml THK p. H 8. 2 n OD 415 vs DW = ODA 2 58
Microcolumn chromatography Procedure n Mix 10 ml remaining diluted hemolysate with 20 ml DW n OD 415 vs DW = ODTotal Calculate n Hb. A 2=(ODA 2 / ODTotal x 5) x 100 59

Microcolumn chromatography 60
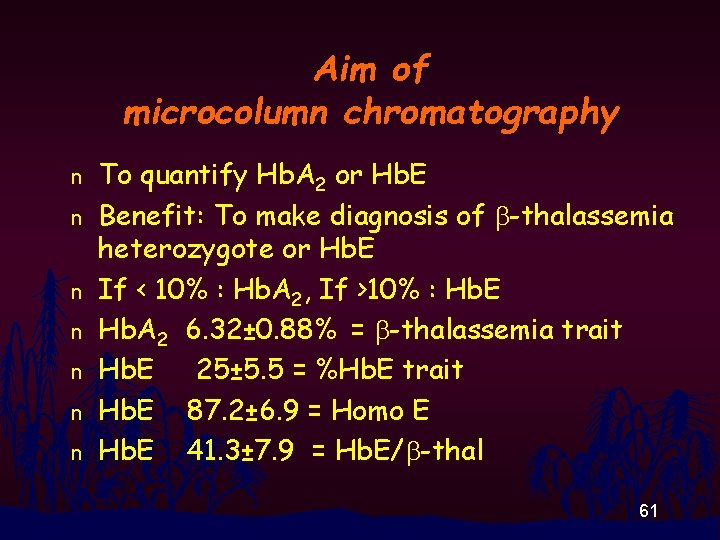
Aim of microcolumn chromatography n n n n To quantify Hb. A 2 or Hb. E Benefit: To make diagnosis of b-thalassemia heterozygote or Hb. E If < 10% : Hb. A 2, If >10% : Hb. E Hb. A 2 6. 32± 0. 88% = b-thalassemia trait Hb. E 25± 5. 5 = %Hb. E trait Hb. E 87. 2± 6. 9 = Homo E Hb. E 41. 3± 7. 9 = Hb. E/b-thal 61

Alkali denaturation test Principle n Hb F is resistant to alkali treatment while other Hbs are not and denatured. 62

Alkali denaturation test Reagent: n Cyanide solution, 1. 2 N Na. OH, Sat Amm. Sulfate Equipmet : n Test tubes (13 x 100), Stop watch, Funnel, Whatman No 1 filter paper, Spectrophotometer 63

Alkali denaturation test Procedure n Mix 200 ul hemolysate with 3. 8 ml cyanide solution Cyan. Hb n Treat 2. 8 ml cyan. Hb with 200 ul 1. 2 N Na. Cl for 2 min exactly n Stop reaction with 2. 0 ml Sat. Amm. Sulfate and filter through Whatman No 1 n Read OD 540 of filtrate = ODFiltrate n Mix 400 ul Cyan. Hb with 6. 75 ml DW n Read OD 540 of total = ODTotal n Calculate Hb. F(%) = ODFiltrate x 100/ODTotal x 10. 01 n OR Hb. F(%) =[ ODFiltrate/ODTotal] x 10 64

Alkali Denaturation Test n n Normal 0. 2 -2. 0% High in b-thalassemia disease, HPFH, db-thalassemia Normal or slightly high in b-thal trait : Hb. E trait and Homo E Hb Bart’s is also resistant to alkali denaturation. 65

Alkali denaturation test 66
67

Final product (OD 540) Total Filtrate 68

Acid Elution Test • Hb. F also resists to acid treatment while others (except Hb Bart’s) do not. • F cell : normal or slightly high in b-thal trait and heterocellular HPFH • F cell : 10 -100% in b-thalassemia 69

Acid Elution Test n n n n Make a thin blood film, let it air-dry Fix the smear in 80% Et. OH for 2 min exactly Dip the fixed smear into 0. 1% Amido Black solution in 80% Et. OH p. H 1. 5 -2. 0 for 2 min exactly Wash the stained blood smear with flushing tap water Let it air-dry Look for F cell (stained deep blue) under oilimmersion power Report in % (in 1, 000 rbc examined), but if few F cells are seen, report as number per HPF 70
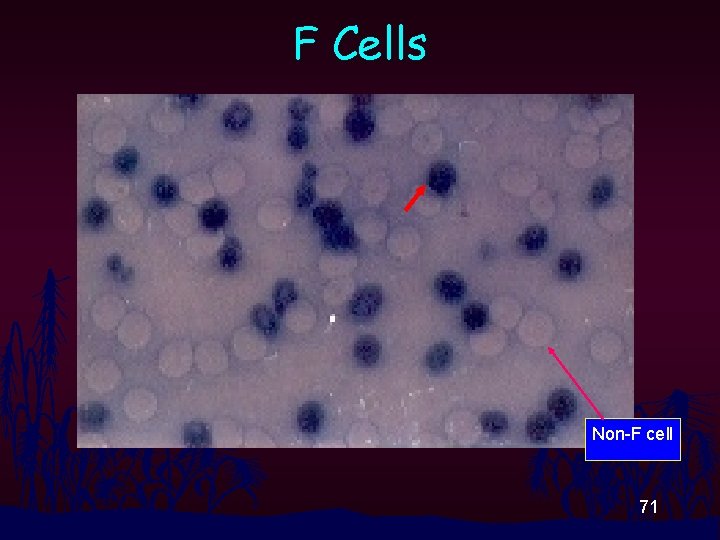
F Cells Non-F cell 71

Hb identification by HPLC Principle n Hb is amphoteric molecule and changes net charge according to p. H of medium. n If p. H < PI, net charge becomes positive (cation ) and different Hbs have different positive charge. n HPLC separation of Hbs is based on cation exchange chromatography n Stationary phase is negatively charged by functional group, e. g. polyaspatic acid. n Mobile phase is buffer with p. H lower than p. I of Hbs n Order of Hbs : Bart’s, H, F, A, A 2/E according to RT 72
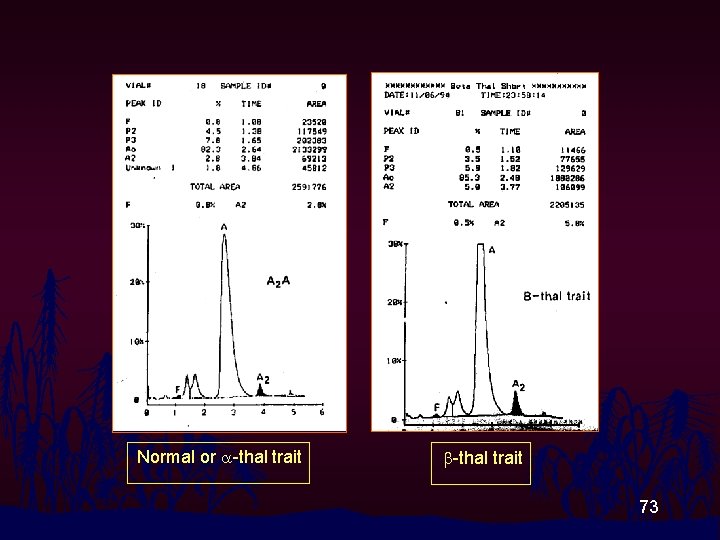
Normal or a-thal trait b-thal trait 73
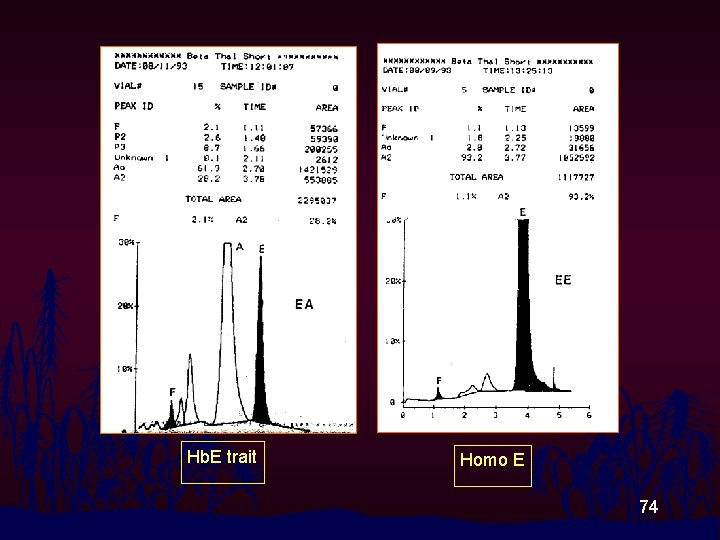
Hb. E trait Homo E 74
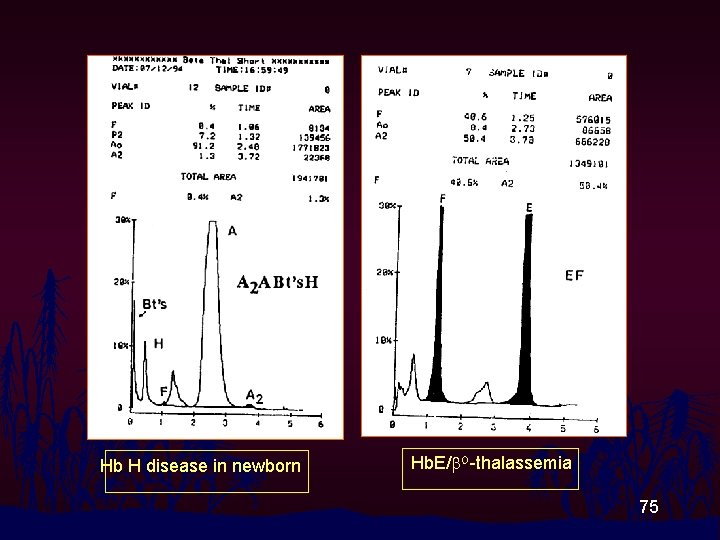
Hb H disease in newborn Hb. E/b. O-thalassemia 75
Immunologic Demonstration of Hemoglobin • Polyclonal and monoclonal Abs for Hbs are produced aiming to detect Hbs in heterozygous state. • Anti-Hb Bart’s for a-thalassemia 1 heterozygote • Anti-Hb Bart’s plus anti z-globin chain for athalassemia 1 heterozygote (SEA type) • Anti Hb. E for Hb. E heterozygote • Anti Hb. A 2 for b-thalassemia heterozygote • Detection techniques : RIA, ELISA, Immunochromatography (Strip test) Immunofluorescence staining and examine using fluoromicroscope or flow cytometer. 76
DNA Analysis • DNA was released from nucleated cells ; white blood cells. • Polymerase Chain Reaction (PCR) to amplify globin gene fragment • Mutation detection by: electrophoresis, hybridization or gene sequencing

Thank You ALL
- Slides: 78