Laboratory Detection and Pure Rotational Spectrum of VCl

Laboratory Detection and Pure Rotational Spectrum of VCl+ by Velocity Modulation Spectroscopy De. Wayne T. Halfen and Lucy M. Ziurys Department of Chemistry Department of Astronomy Steward Observatory Arizona Radio Observatory University of Arizona June 21, 2004
Buy one, get one free • VCl+ spectrum appears along with that of VCl • Factor of 5 weaker than VCl • NO past work on this ion • Electronic ground state unknown • 4 D or 4 F or even 4 S r r • First laboratory detection and pure rotational spectrum

Gas-Phase Synthesis of VCl+ • Add VCl 4 – Pressure: 1 -2 m. Torr • 20 m. Torr Ar gas also added • AC discharge – 200 W at 600 W
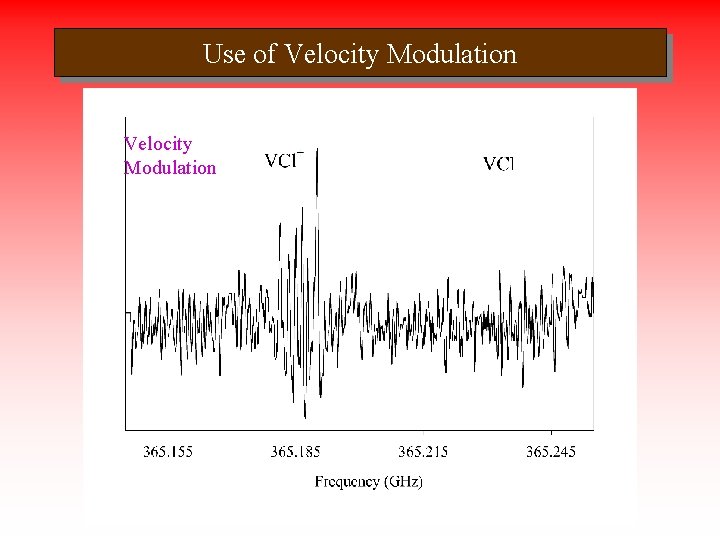
Use of Velocity Modulation Source Velocity Modulation
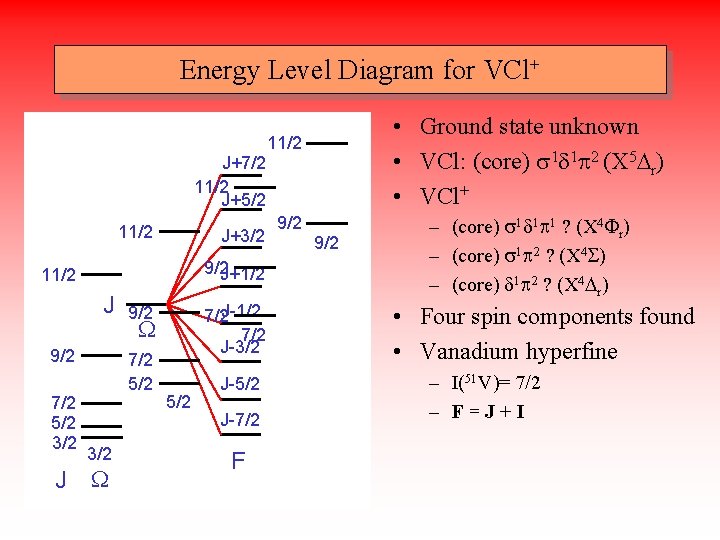
Energy Level Diagram for VCl+ J+7/2 11/2 J+5/2 11/2 J+3/2 9/2 J+1/2 11/2 J 9/2 7/2 5/2 3/2 W 7/2 5/2 3/2 J W 7/2 J-1/2 7/2 J-3/2 9/2 5/2 J-7/2 F • Ground state unknown • VCl: (core) s 1 d 1 p 2 (X 5 Dr) • VCl+ 11/2 9/2 – (core) s 1 d 1 p 1 ? (X 4 Fr) – (core) s 1 p 2 ? (X 4 S) – (core) d 1 p 2 ? (X 4 Dr) • Four spin components found • Vanadium hyperfine – I(51 V)= 7/2 – F=J+I
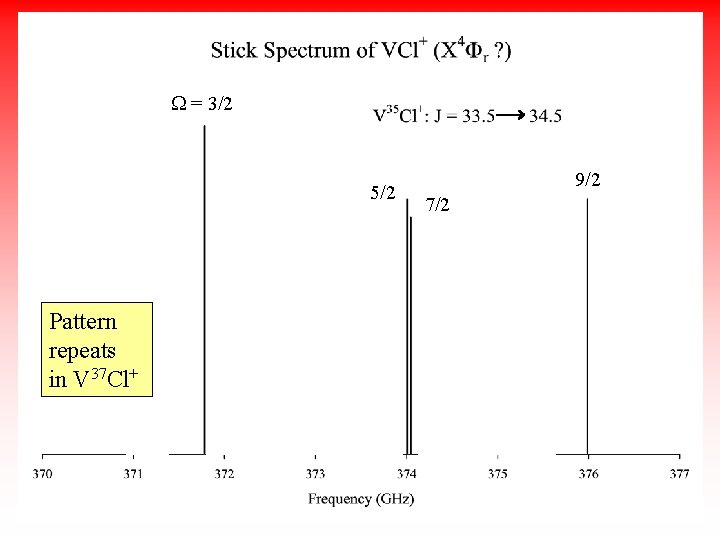
W = 3/2 5/2 Pattern repeats in V 37 Cl+ 5/2 7/2 9/2
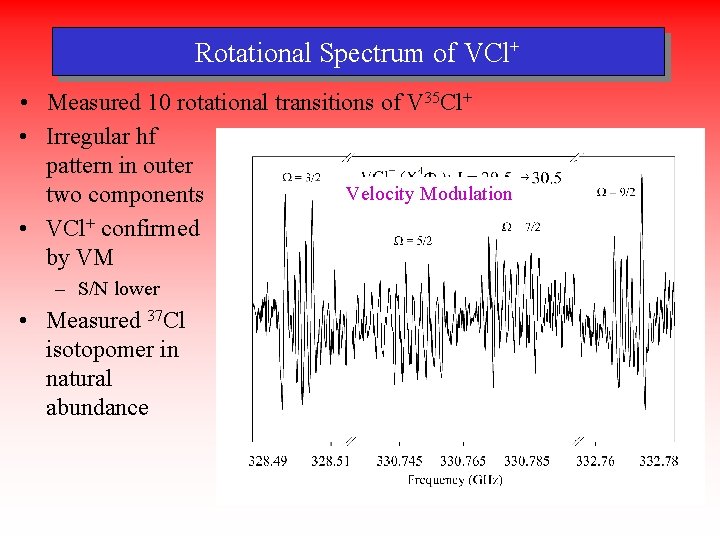
Rotational Spectrum of VCl+ • Measured 10 rotational transitions of V 35 Cl+ • Irregular hf pattern in outer Velocity Source Modulation two components • VCl+ confirmed by VM – S/N lower • Measured 37 Cl isotopomer in natural abundance
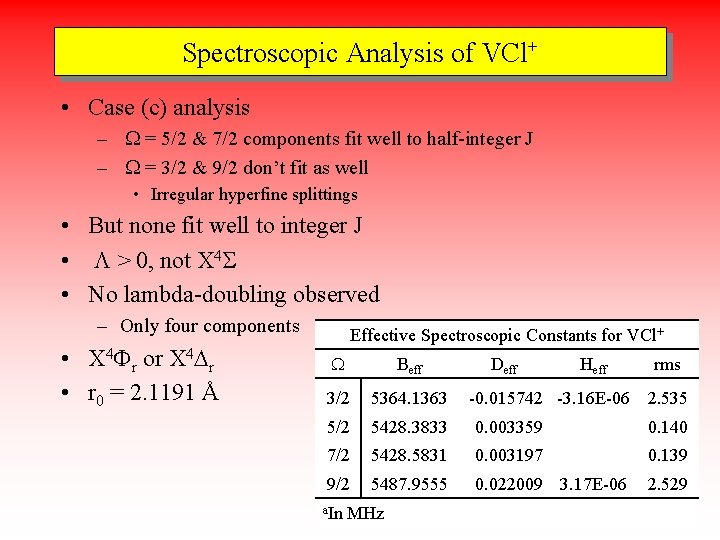
Spectroscopic Analysis of VCl+ • Case (c) analysis – W = 5/2 & 7/2 components fit well to half-integer J – W = 3/2 & 9/2 don’t fit as well • Irregular hyperfine splittings • But none fit well to integer J • L > 0, not X 4 S • No lambda-doubling observed – Only four components • X 4 Fr or X 4 Dr • r 0 = 2. 1191 Å Effective Spectroscopic Constants for VCl+ W Beff 3/2 5364. 1363 5/2 5428. 3833 0. 003359 0. 140 7/2 5428. 5831 0. 003197 0. 139 9/2 5487. 9555 0. 022009 3. 17 E-06 2. 529 a. In MHz Deff Heff rms -0. 015742 -3. 16 E-06 2. 535
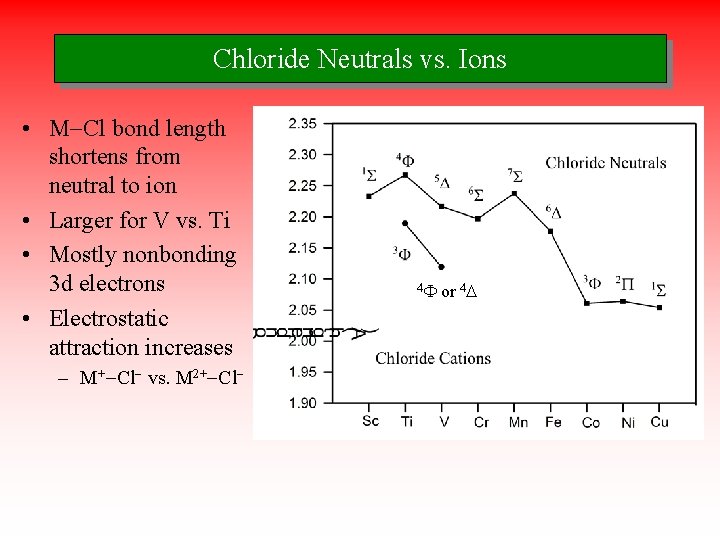
Chloride Neutrals vs. Ions • M-Cl bond length shortens from neutral to ion • Larger for V vs. Ti • Mostly nonbonding 3 d electrons • Electrostatic attraction increases – M+-Cl- vs. M 2+-Cl- 4 F or 4 D
Future Work • Finish measurements of VCl+ • Determine ground state conclusively • Measure spectra of more molecular ions – Fe. CO+ (X 4 S+) – Al. NC+ (X 2 S+)
Acknowledgements • • Prof. Lucy Ziurys Dr. Aldo Apponi Dr. Chandra Savage Michael Flory Alexandra Janczyk Stefanie Milam Emily Tenenbaum Edward Post • NSF • NASA
- Slides: 11