Labile and inert metal ions Kinetic effects Water
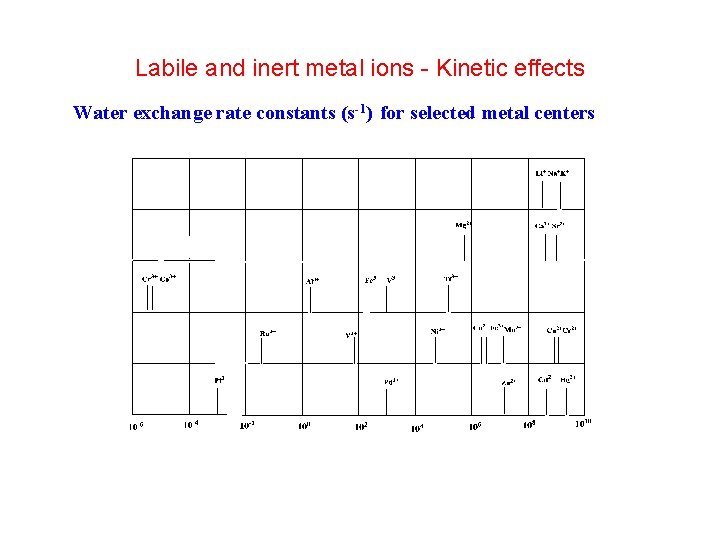
Labile and inert metal ions - Kinetic effects Water exchange rate constants (s-1) for selected metal centers
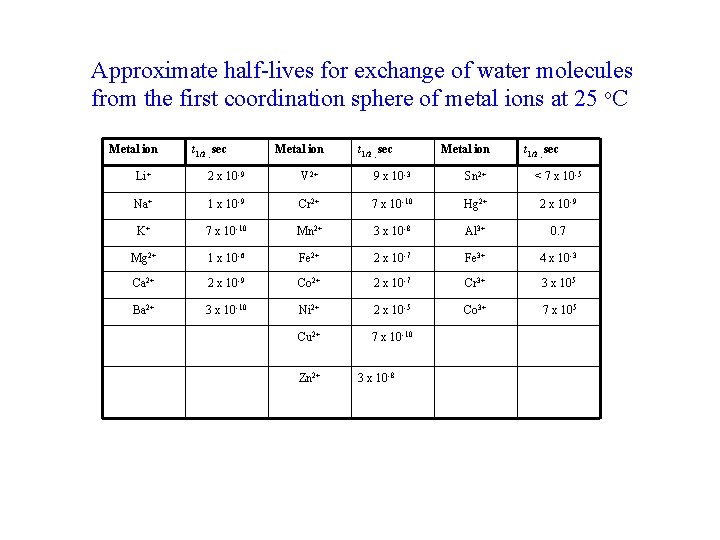
Approximate half-lives for exchange of water molecules from the first coordination sphere of metal ions at 25 o. C Metal ion t 1/2 , sec Li+ 2 x 10 -9 V 2+ 9 x 10 -3 Sn 2+ < 7 x 10 -5 Na+ 1 x 10 -9 Cr 2+ 7 x 10 -10 Hg 2+ 2 x 10 -9 K+ 7 x 10 -10 Mn 2+ 3 x 10 -8 Al 3+ 0. 7 Mg 2+ 1 x 10 -6 Fe 2+ 2 x 10 -7 Fe 3+ 4 x 10 -3 Ca 2+ 2 x 10 -9 Co 2+ 2 x 10 -7 Cr 3+ 3 x 105 Ba 2+ 3 x 10 -10 Ni 2+ 2 x 10 -5 Co 3+ 7 x 105 Cu 2+ 7 x 10 -10 Zn 2+ 3 x 10 -8
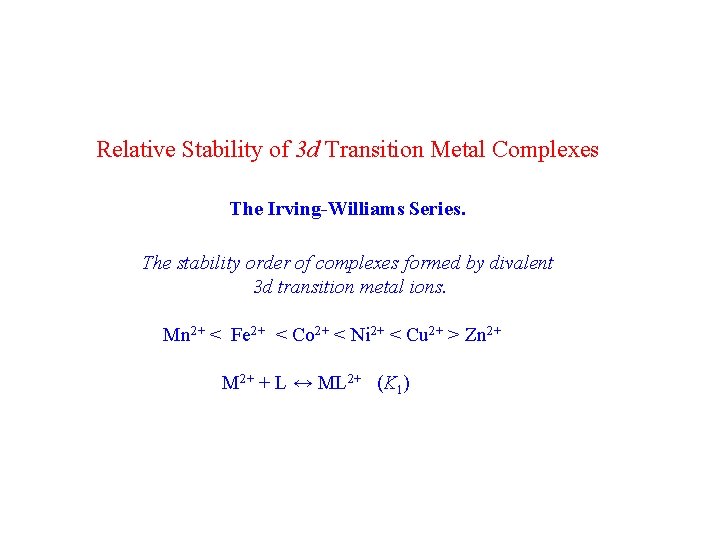
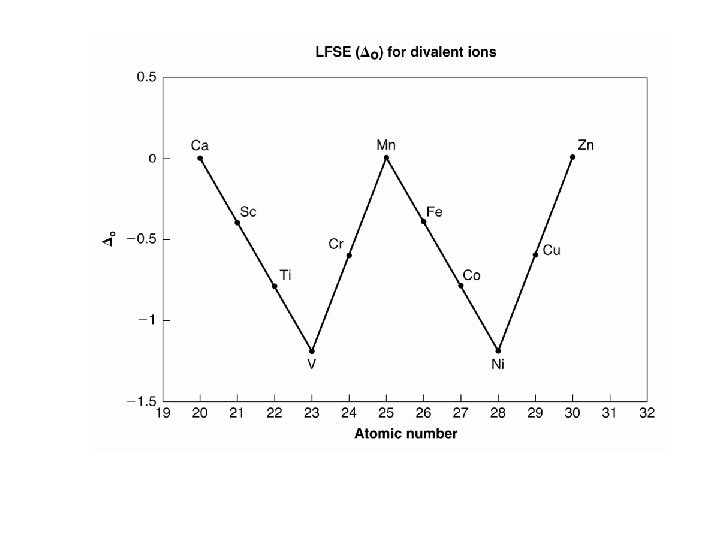
Relative Stability of 3 d Transition Metal Complexes The Irving-Williams Series. The stability order of complexes formed by divalent 3 d transition metal ions. Mn 2+ < Fe 2+ < Co 2+ < Ni 2+ < Cu 2+ > Zn 2+ M 2+ + L ↔ ML 2+ (K 1)
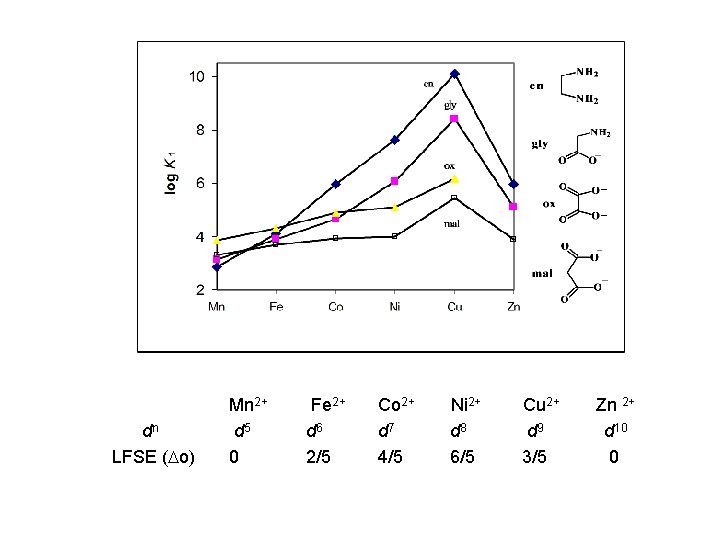
dn LFSE ( o) Mn 2+ d 5 0 Fe 2+ d 6 2/5 Co 2+ d 7 4/5 Ni 2+ d 8 6/5 Cu 2+ d 9 3/5 Zn 2+ d 10 0
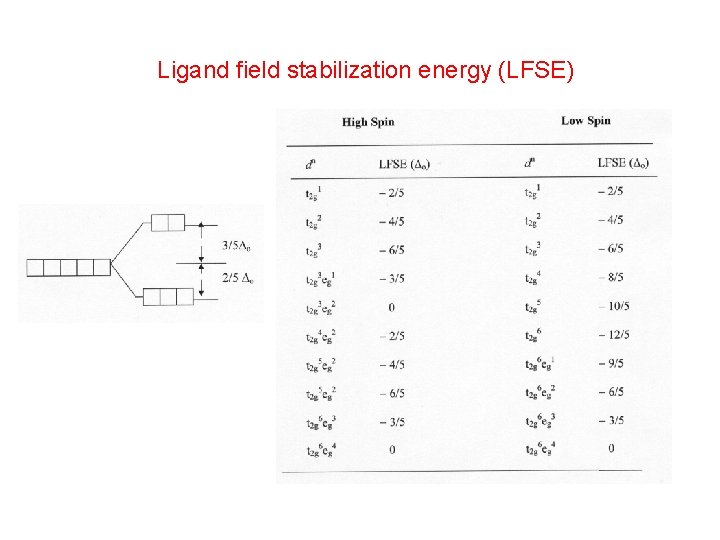
Ligand field stabilization energy (LFSE)
![M 2+(g) + n. H 2 O [M(H 2 O)6]2+ Hhydration M 2+(g) + n. H 2 O [M(H 2 O)6]2+ Hhydration](http://slidetodoc.com/presentation_image_h/a49a8a619e8a19330cb8b1a6b11f2e3a/image-7.jpg)
M 2+(g) + n. H 2 O [M(H 2 O)6]2+ Hhydration
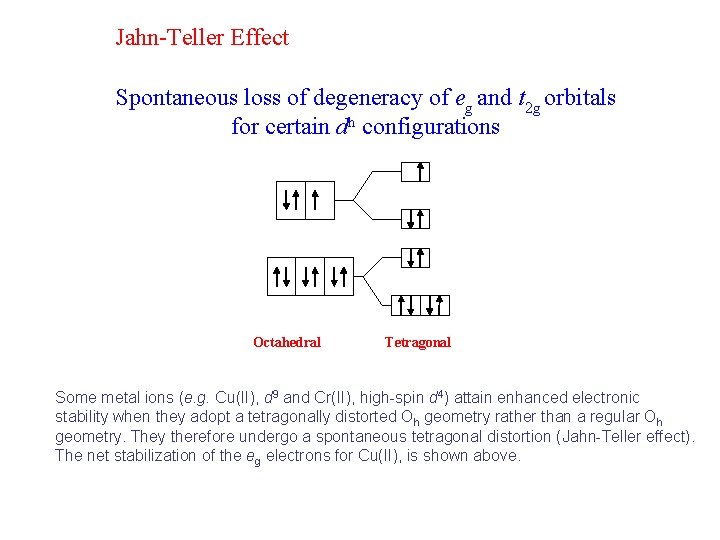
Jahn-Teller Effect Spontaneous loss of degeneracy of eg and t 2 g orbitals for certain dn configurations Octahedral Tetragonal Some metal ions (e. g. Cu(II), d 9 and Cr(II), high-spin d 4) attain enhanced electronic stability when they adopt a tetragonally distorted Oh geometry rather than a regular Oh geometry. They therefore undergo a spontaneous tetragonal distortion (Jahn-Teller effect). The net stabilization of the eg electrons for Cu(II), is shown above.
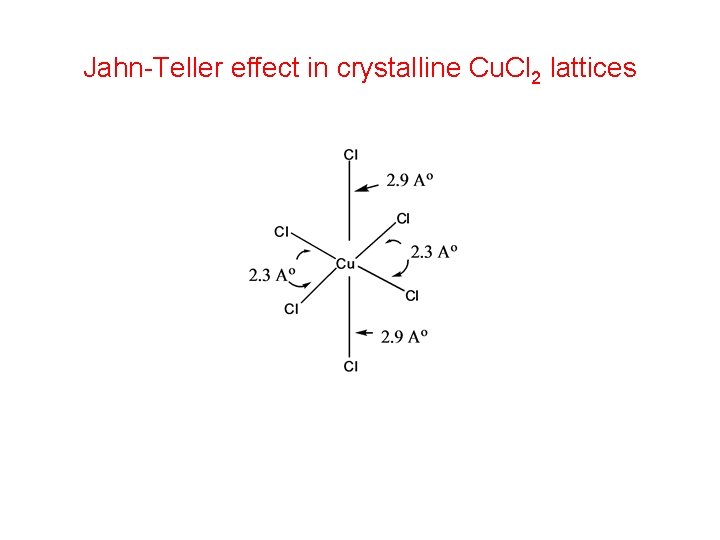
Jahn-Teller effect in crystalline Cu. Cl 2 lattices
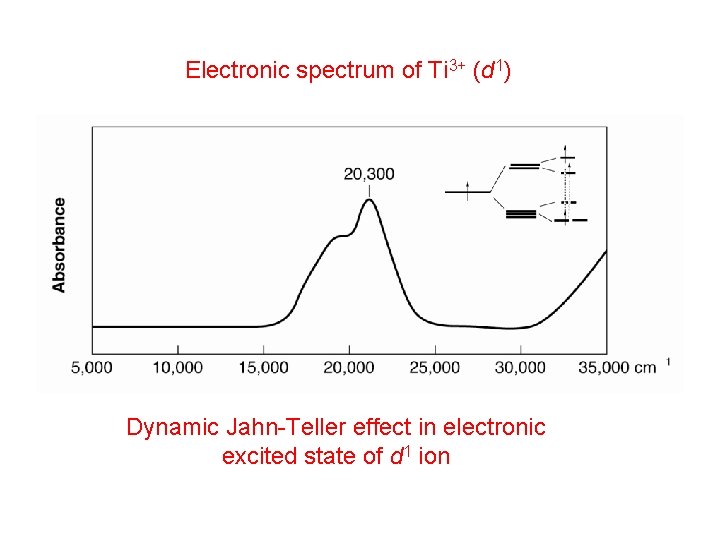
Electronic spectrum of Ti 3+ (d 1) Dynamic Jahn-Teller effect in electronic excited state of d 1 ion
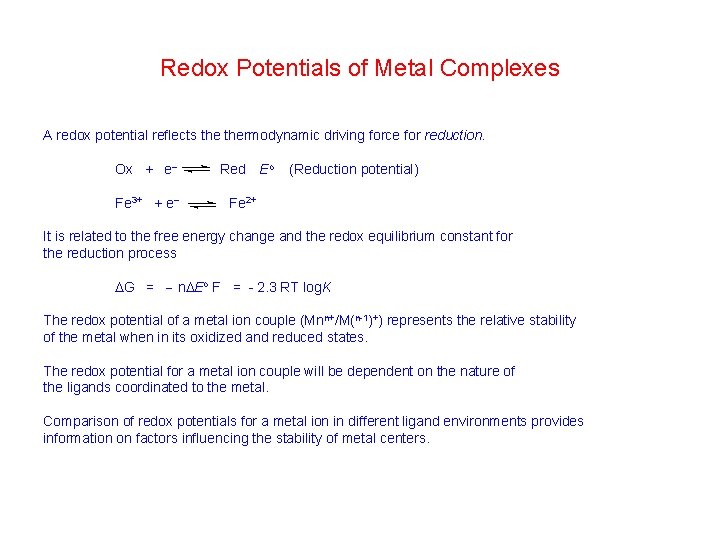
Redox Potentials of Metal Complexes A redox potential reflects thermodynamic driving force for reduction. Ox + e Fe 3+ + e Red Eo (Reduction potential) Fe 2+ It is related to the free energy change and the redox equilibrium constant for the reduction process G = n Eo F = - 2. 3 RT log. K The redox potential of a metal ion couple (Mnn+/M(n-1)+) represents the relative stability of the metal when in its oxidized and reduced states. The redox potential for a metal ion couple will be dependent on the nature of the ligands coordinated to the metal. Comparison of redox potentials for a metal ion in different ligand environments provides information on factors influencing the stability of metal centers.
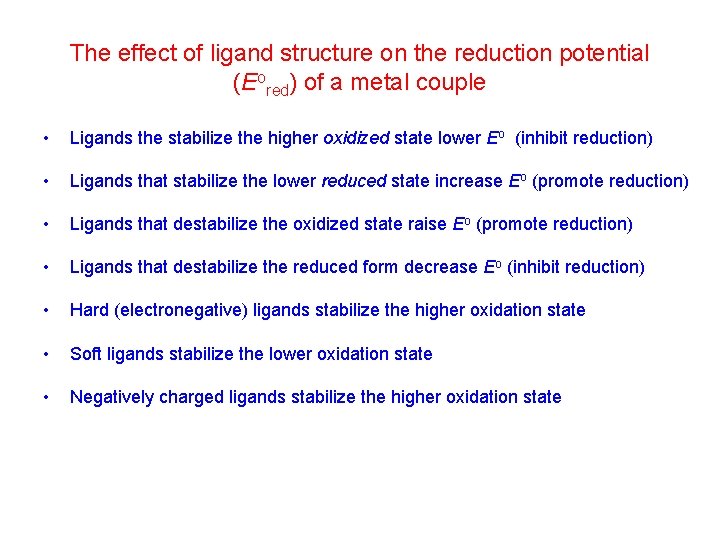
The effect of ligand structure on the reduction potential (Eored) of a metal couple • Ligands the stabilize the higher oxidized state lower Eo (inhibit reduction) • Ligands that stabilize the lower reduced state increase Eo (promote reduction) • Ligands that destabilize the oxidized state raise Eo (promote reduction) • Ligands that destabilize the reduced form decrease Eo (inhibit reduction) • Hard (electronegative) ligands stabilize the higher oxidation state • Soft ligands stabilize the lower oxidation state • Negatively charged ligands stabilize the higher oxidation state
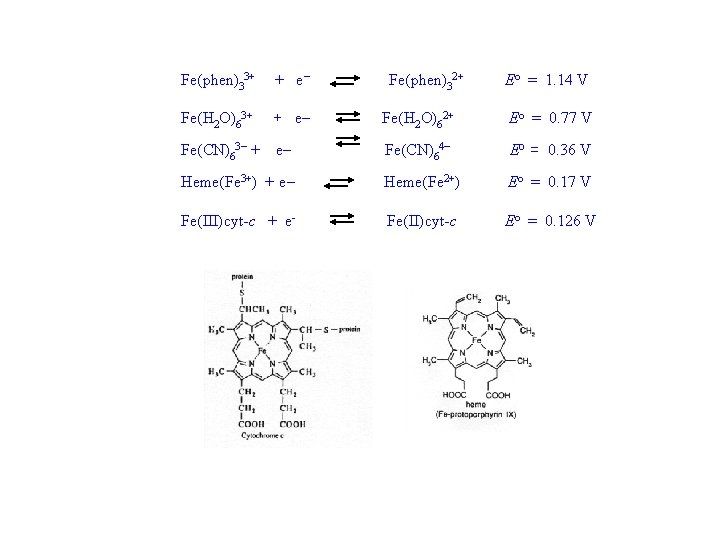
Fe(phen)33+ + e Fe(H 2 O)62+ Eo = 0. 77 V Fe(CN)63 + e Fe(CN)64 Eo = 0. 36 V Heme(Fe 3+) + e Heme(Fe 2+) Eo = 0. 17 V Fe(III)cyt-c + e- Fe(II)cyt-c Eo = 0. 126 V Fe(phen)32+ Eo = 1. 14 V

• Soft 1, 10 -phenanthroline stabilizes Fe in the softer lower Fe(II) state - i. e. it provides greater driving force for reduction of Fe(III) to Fe(II) • Hard oxygen in H 2 O favors the harder Fe(III) state. - resulting in a lower driving force for reduction of Fe(III) to Fe(II) • Negatively charged CN- favors the higher Fe(III) oxidation state (hard - hard interaction) - i. e. it provides a lower driving force for reduction.
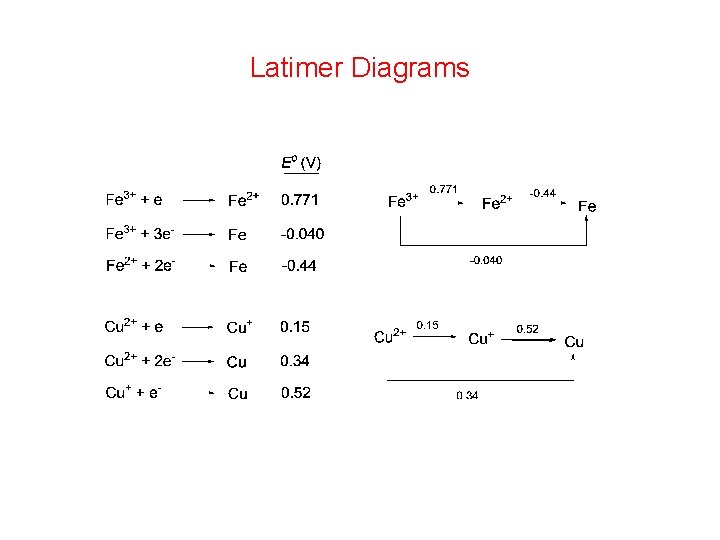
Latimer Diagrams
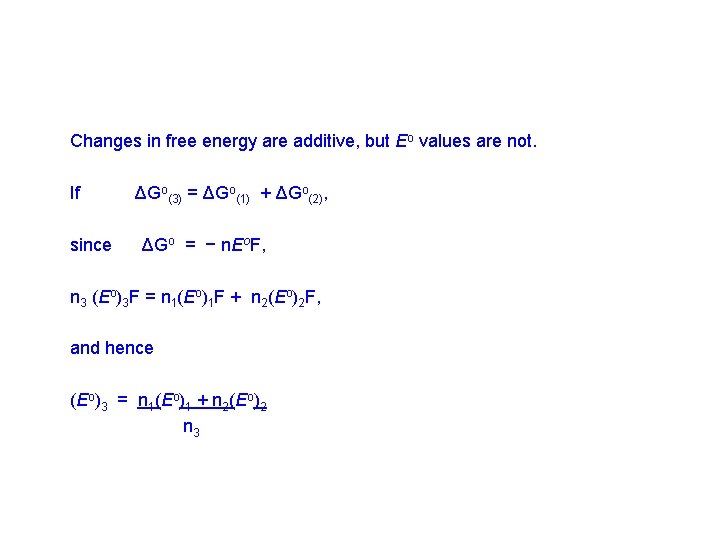
Changes in free energy are additive, but Eo values are not. If since ΔGo(3) = ΔGo(1) + ΔGo(2), ΔGo = − n. Eo. F, n 3 (Eo)3 F = n 1(Eo)1 F + n 2(Eo)2 F, and hence (Eo)3 = n 1(Eo)1 + n 2(Eo)2 n 3
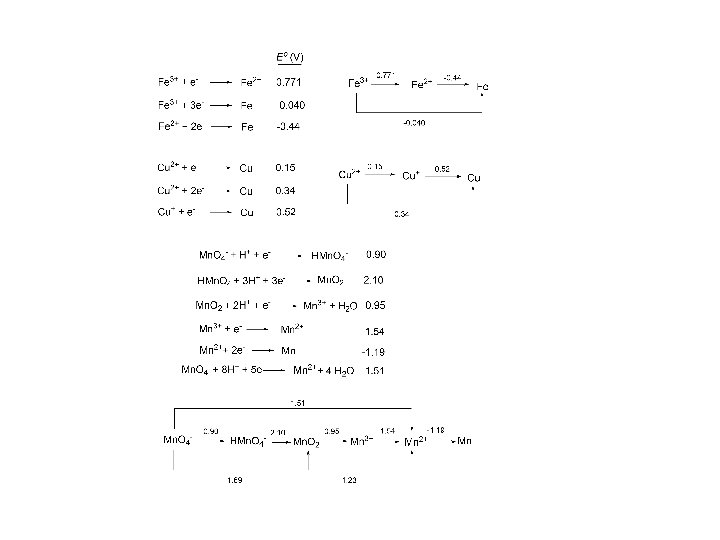

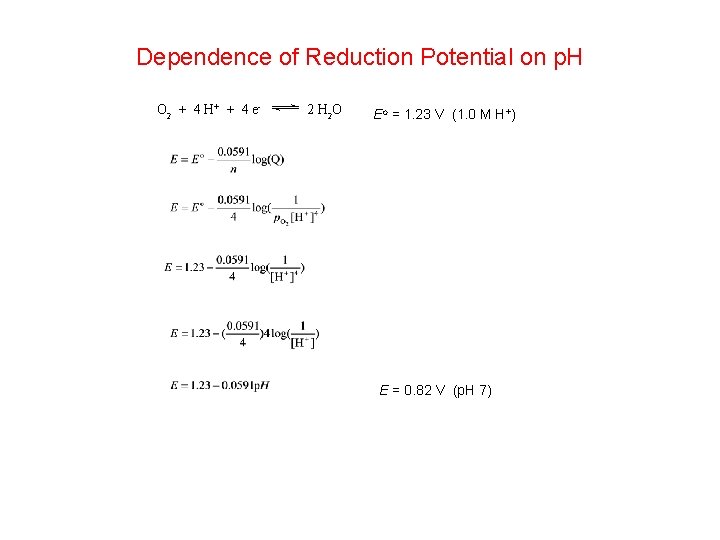
Dependence of Reduction Potential on p. H O 2 + 4 H + + 4 e - 2 H 2 O Eo = 1. 23 V (1. 0 M H+) E = 0. 82 V (p. H 7)
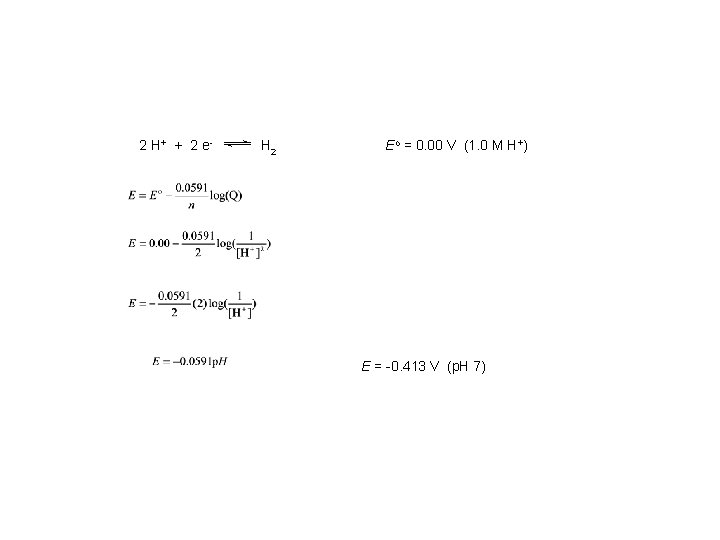
2 H+ + 2 e - H 2 Eo = 0. 00 V (1. 0 M H+) E = -0. 413 V (p. H 7)
- Slides: 20