Lab Identifying Elements Compounds and Mixtures Directions Take
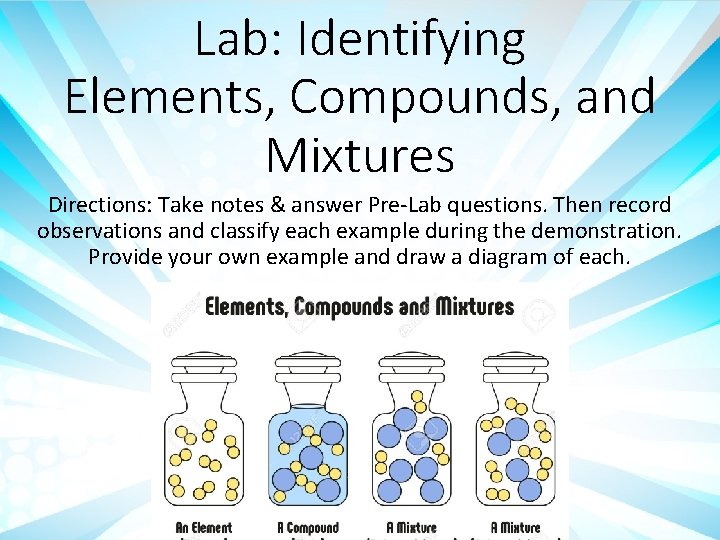
Lab: Identifying Elements, Compounds, and Mixtures Directions: Take notes & answer Pre-Lab questions. Then record observations and classify each example during the demonstration. Provide your own example and draw a diagram of each.

Element • Pure substance; Made of ONE kind of atom (found on periodic table). • Can not be separated into any simpler form chemically OR physically.
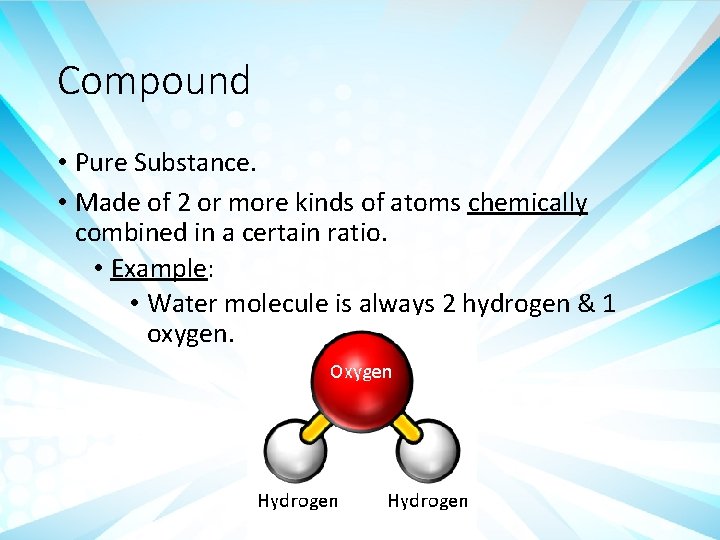
Compound • Pure Substance. • Made of 2 or more kinds of atoms chemically combined in a certain ratio. • Example: • Water molecule is always 2 hydrogen & 1 oxygen.
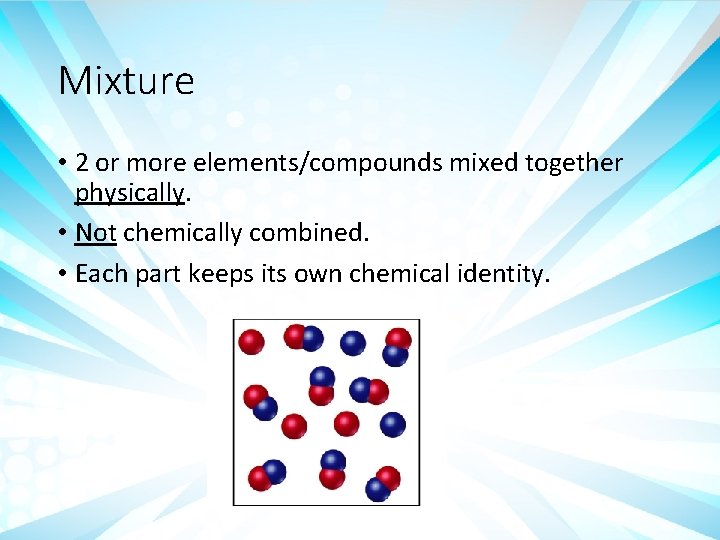
Mixture • 2 or more elements/compounds mixed together physically. • Not chemically combined. • Each part keeps its own chemical identity.

Pre-Lab Questions Answer on your paper. 1. What is the difference between an atom and a compound? 2. Why is an element the simplest form of matter? 3. How is the way a mixture is combined DIFFERENT from how a compound is combined? 4. What is easier to separate, a mixture or a compound? Why? 5. Which can be found on the periodic table: elements, compounds, or mixtures?
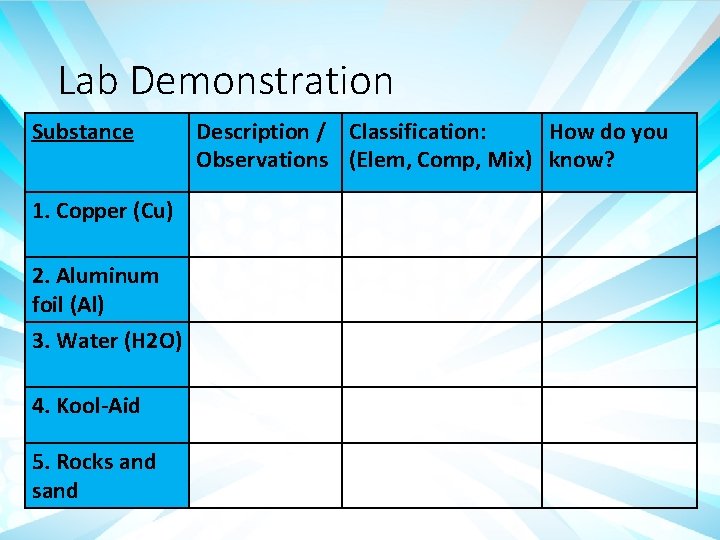
Lab Demonstration Substance 1. Copper (Cu) 2. Aluminum foil (Al) 3. Water (H 2 O) 4. Kool-Aid 5. Rocks and sand Description / Classification: How do you Observations (Elem, Comp, Mix) know?
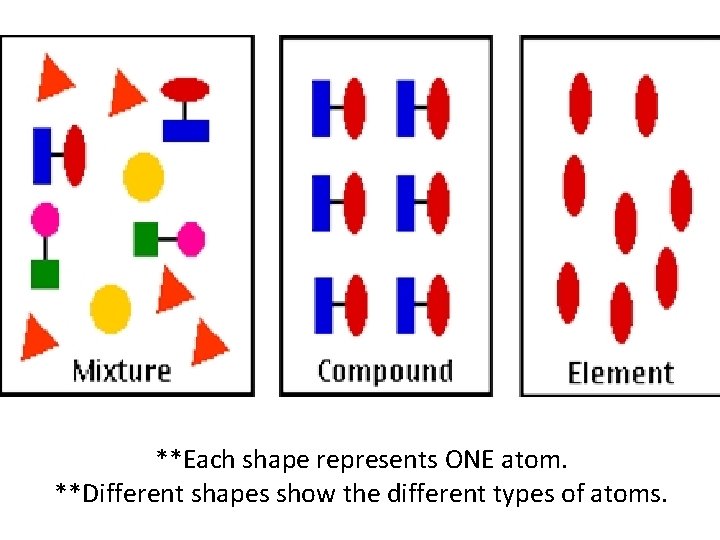
**Each shape represents ONE atom. **Different shapes show the different types of atoms.
- Slides: 7