LAB EQUIPMENT SPECTROSCOPY Studying what occurs with matter

LAB EQUIPMENT

SPECTROSCOPY • Studying what occurs with matter when it is interacted with electromagnetic radiation • Our colorimeters are spectroscopes. • A beam of light is passed through a solution. Some of that light may or may not be absorbed by the solution. • The light being absorbed is due to resonance in the electronic vibrations, the electrons in the molecules that make up the bonds can vibrate.

RESONANCE • Materials have a natural frequency, that is the frequency that it will vibrate at the easiest. • You can shatter a glass if you can sing a note exactly at the natural frequency of the glass. • Flick the glass and listen for the sound it makes. Sing a note exactly at that sound and those sound waves at the right frequency will be absorbed by the glass and cause it to vibrate. The others pass by.

SPECTROSCOPY • The light resonating with electrons is what occurs with spectroscopy. • When light is emitted though, some will resonate, shake bend, twist different electrons at different sites. • This is the energy that is absorbed
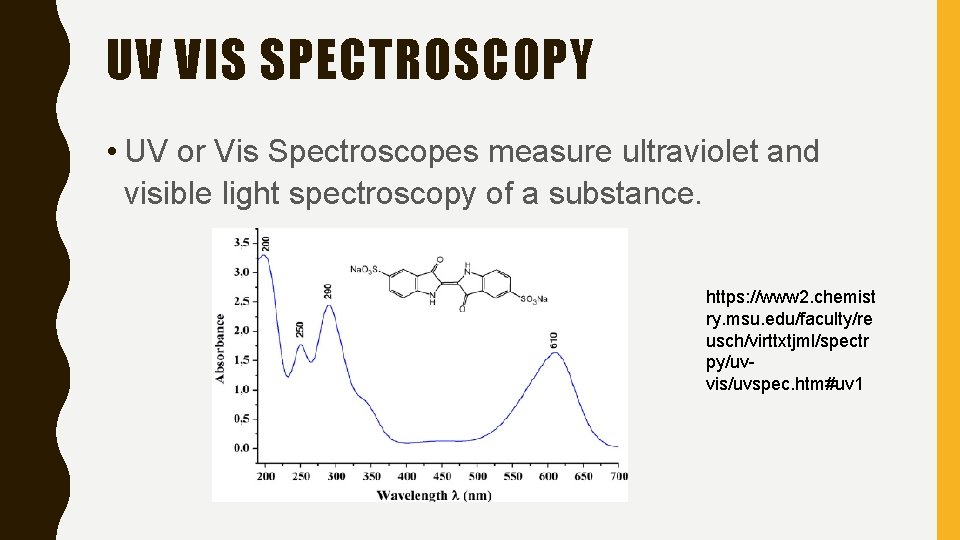
UV VIS SPECTROSCOPY • UV or Vis Spectroscopes measure ultraviolet and visible light spectroscopy of a substance. https: //www 2. chemist ry. msu. edu/faculty/re usch/virttxtjml/spectr py/uvvis/uvspec. htm#uv 1

FOURIER TRANSFORM INFRARED SPECTROSCOPY, FTIR • This uses a method to measure the absorption of infrared light. • A Fourier transform is a mathematical process required to convert the raw data into a spectrum. • Instead of shining a monochromatic light beam into a source, a beam containing many frequencies is emitted into the substance. This is repeated with many different beams containing different combination of light frequencies. • A computer is used to work backwards from this data to infer which light beams are absorbed using a common

FTIR • Although a bit counterintuitive. FTIR lessons a common problem in IR spectrometry, noise. • In essence it is difficult to get a perfect monochromatic IR light, by testing many frequencies of infrared light at once the signal to noise ratio is much higher.
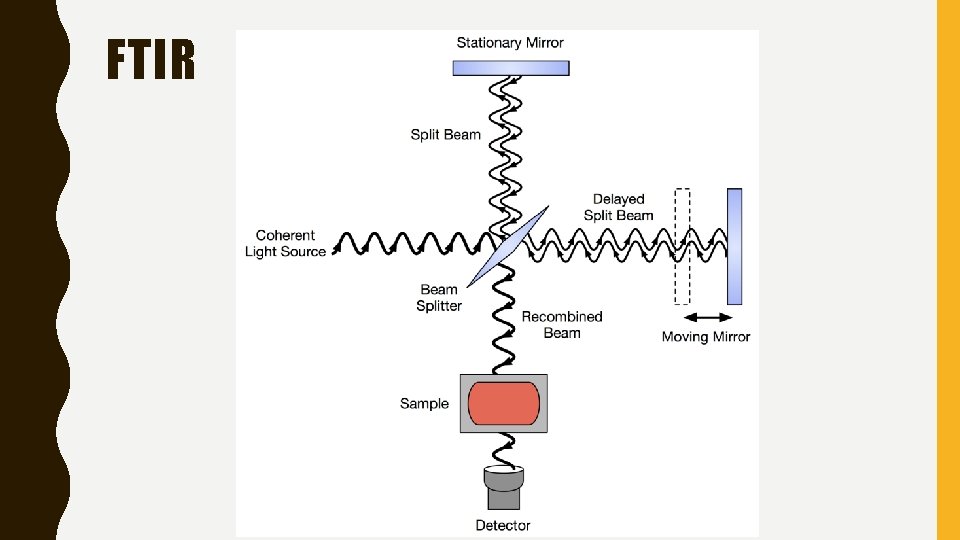
FTIR
MICROPROBE ATTENUATED TOTAL REFLECTANCE (ATR-FTIR) • Commonly used in conjunction with FTIR, ATR is an attachment to the FTIR machine. • An ATR crystal uses total internal reflection to create an evanescent wave, a wave that doesn’t propagate, that comes in contact with the sample.

MICROSCOPY • Study of using tools to see objects too small to be seen with the naked eye. • A standard compound light microscope uses lenses to magnify light images. • Diffraction limits magnification to approximately 1500 x
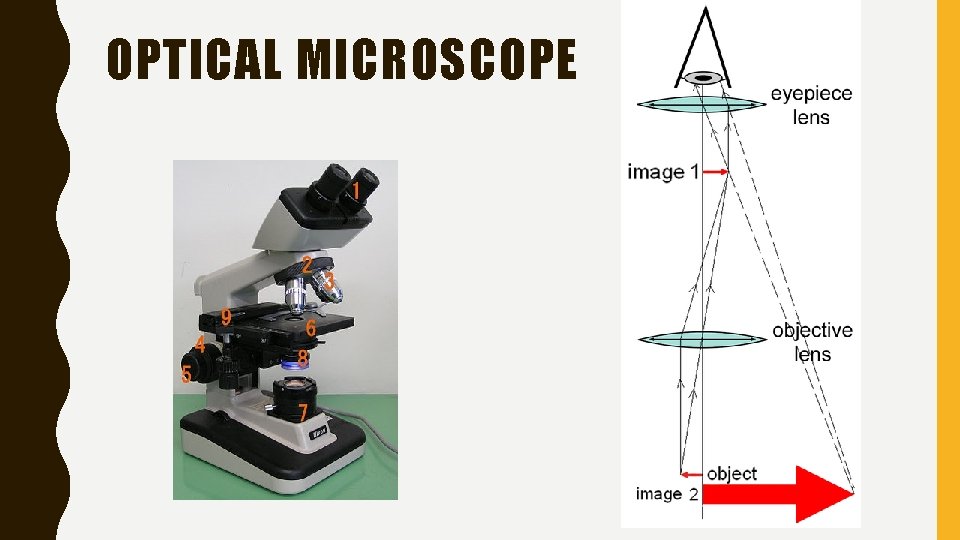
OPTICAL MICROSCOPE

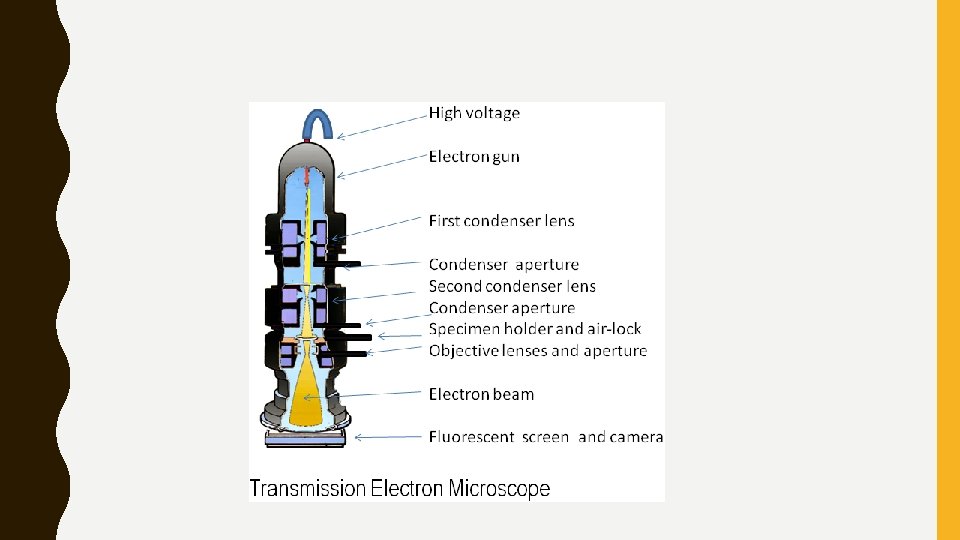
ELECTRON MICROSCOPES • Works in a very similar fashion to the optical microscope, however, instead of visible light it uses a stream of electrons. • Electrons have a wavelength 100, 000 x shorter than visible light photons. • This allows for magnifications of up to 10, 000 x

SCANNING ELECTRON MICROSCOPE (SEM) • SEM works by probing the surface of an object with a beam of electrons. • Detectors check for deflected electrons. • Color- no electron microscope can produce a color image, only gray scale. Some images are colored afterwards. • Ant head---------
THERMAL GRAVIMETRIC ANALYZER • Thermal Gravimetric Analyzer measures physical characteristics; mass, phase, over a variety of temperatures. • This is used to test thermal stability of several polymers and ceramics. • This tells you what temperatures a material is safe to work under.

DIFFERENTIAL SCANNING CALORIMETER • Thermoanalytical device to measure the amount of heat required to increase the temperature of the sample. • A material is heated next to a substance with a known heat capacity, heat flows between the two to keep the temperature constant.
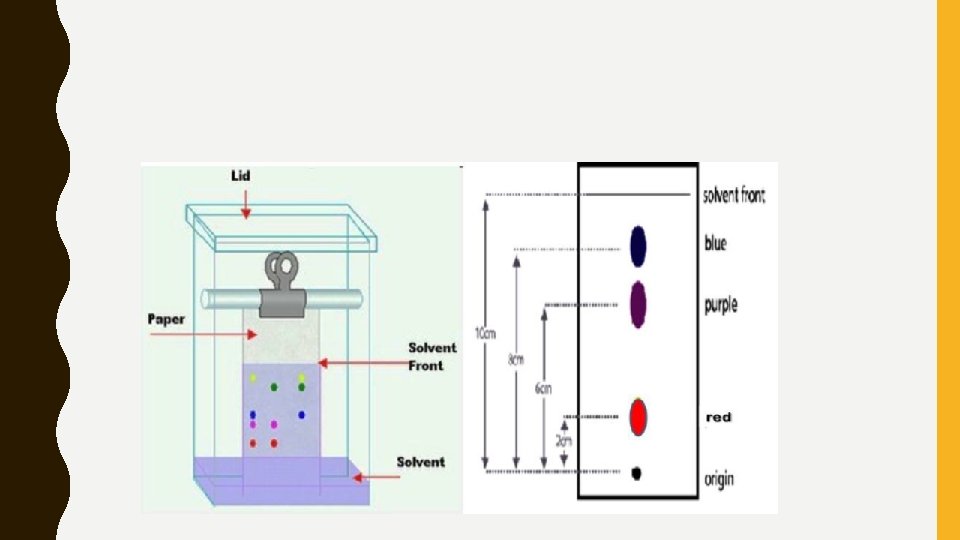
CHROMATOGRAPHY • A method of separating a material by moving a mobile phase through a stationary phase. • A simple example is paper chromatography. • A sample soluble in a solvent is placed on paper. The paper is then place in the solve so that the sample is above the solvent. • The solvent climbs up the paper by capillary action and separates the sample because they have different mobility rates.
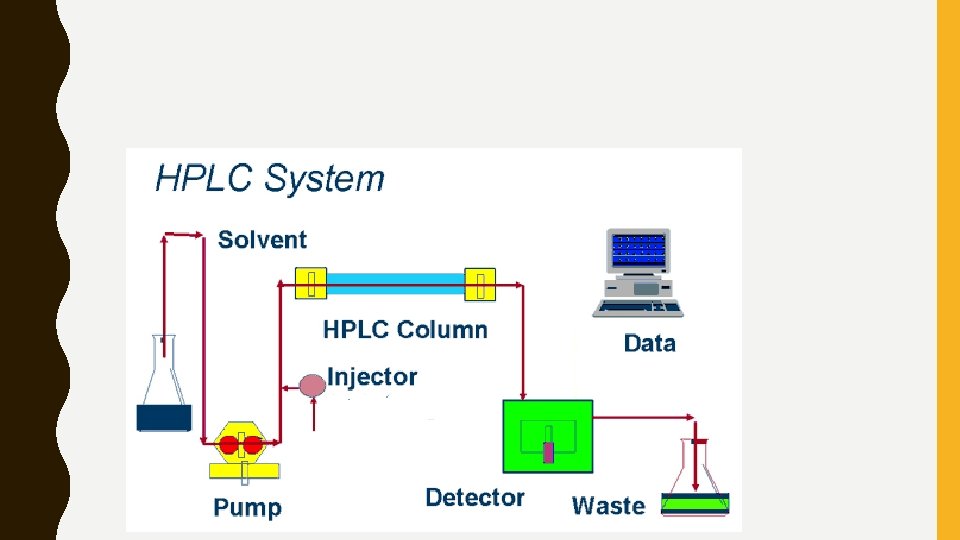
HIGH-PERFORMANCE LIQUID CHROMATOGRAPHY (HPLC) • Works is a manner similar to regular chromatography, except the liquid mobile state is pressurized by a pump. • This forces the mobile state through the stationary phase.

OTHER EQUIPMENT • Particle size analyzer • Dynamic mechanical analyzer- applies forces to test
- Slides: 21