Lab Activity 1 Carbohydrates IUG 2015 Dr Tarek

Lab Activity 1: Carbohydrates IUG, 2015 Dr. Tarek M Zaida

Background • A carbohydrate is an organic compound with the general formula Cm(H 2 O)n, that is, consists only of carbon, hydrogen and oxygen, with the last two in the 2: 1 atom ratio. • Carbohydrates make up the bulk of organic substances on earth and perform numerous roles in living systems. • The carbohydrates (saccharides) are divided into three categories: • Monosaccharides, disaccharides, and polysaccharides.
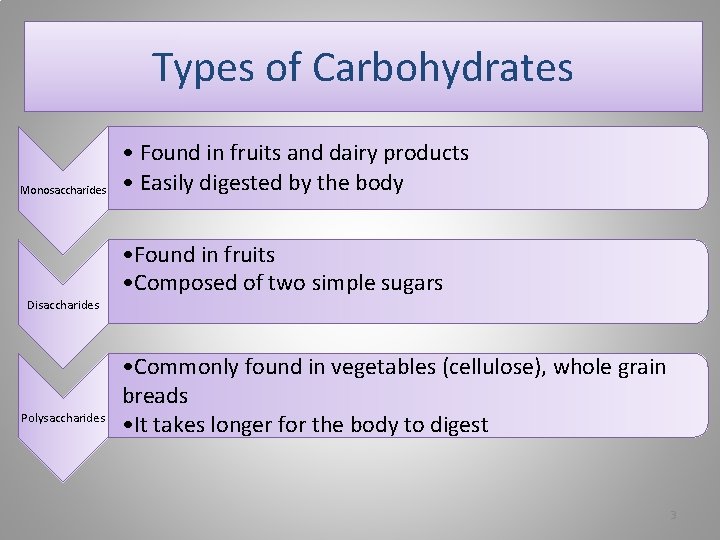
Types of Carbohydrates Monosaccharides Disaccharides Polysaccharides • Found in fruits and dairy products • Easily digested by the body • Found in fruits • Composed of two simple sugars • Commonly found in vegetables (cellulose), whole grain breads • It takes longer for the body to digest 3
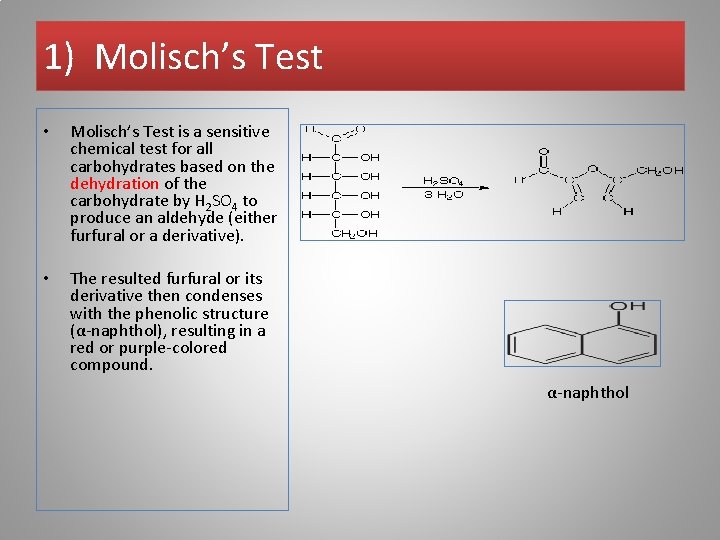
1) Molisch’s Test • Molisch’s Test is a sensitive chemical test for all carbohydrates based on the dehydration of the carbohydrate by H 2 SO 4 to produce an aldehyde (either furfural or a derivative). • The resulted furfural or its derivative then condenses with the phenolic structure (α-naphthol), resulting in a red or purple-colored compound. α-naphthol
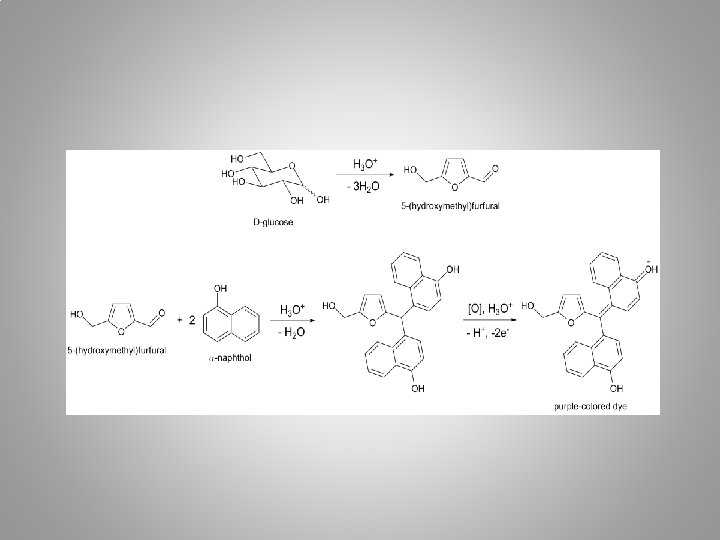
PROCEDURE • Place 2 m. L of a known carbohydrate solution in a test tube, add 1 drop of Molisch’s reagent (10% α-naphthol in ethanol). • Pour 1 -2 m. L of conc. H 2 SO 4 down the side of the test tube, so that it forms a layer at the bottom of the tube. • Observe the color at the interface between two layers and compare your result with a control test.

2) Anthrone Test As in Molisch’s reaction, except for using the anthrone reagent (0. 2% in conc. H 2 SO 4), instead of α-naphthol reagent. Anthrone


Procedure 1. To 5 drops of the carbohydrates solution in a test tube carefully add 2 ml of anthrone reagent, mix thoroughly by swirling, 2. Heat in a boiling water-bath for 3 min. 3. Cool the samples and observe the color formed. The mixture should be of a greenish or bluegreen color of an intensity depending on the amount of carbohydrate present.
- Slides: 9