Lab 8 Sodium Carbonate or Sodium Bicarbonate Objective

Lab 8 Sodium Carbonate or Sodium Bicarbonate? Objective To determine a compound to be either Na 2 CO 3 or Na. HCO 3.
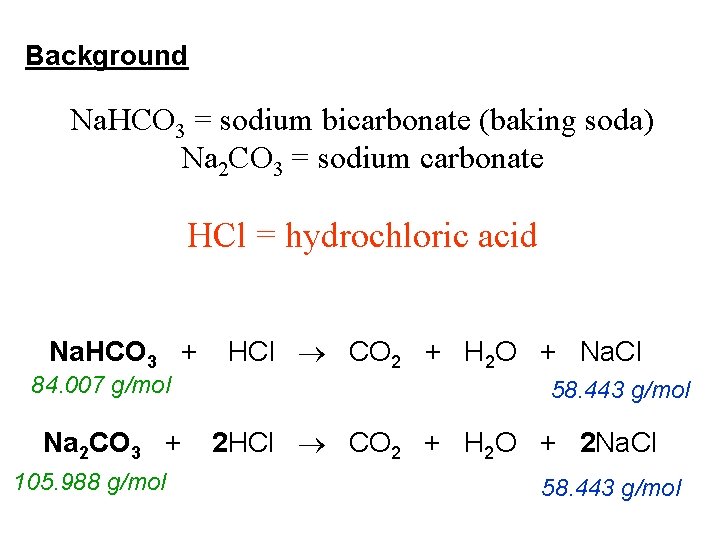
Background Na. HCO 3 = sodium bicarbonate (baking soda) Na 2 CO 3 = sodium carbonate HCl = hydrochloric acid Na. HCO 3 + 84. 007 g/mol Na 2 CO 3 + 105. 988 g/mol HCl CO 2 + H 2 O + Na. Cl 58. 443 g/mol 2 HCl CO 2 + H 2 O + 2 Na. Cl 58. 443 g/mol

Experiment (M 1) Mass of empty evap. dish + watch glass = ____ g (M 2) Mass of dish + watch glass + 1. 00 gram of solid = ____ g Slowly add drops of HCl to the dish until all of the solid has reacted. Slowly evaporate the liquid in the dish with the watch glass on top of the dish. Avoid spattering. Let the evap. dish cool. (M 3) Mass of evap. dish + watch glass + product = ____ g Clean out dish and watch glass.

Analysis 1. What reactant was the excess reactant in this lab? 2. How many grams of the solid did you begin with? 3. Calculate the mass of the Na. Cl that was produced. 4. Convert the mass of the Na. Cl into moles of Na. Cl (keep at least 3 sig figs). You started with 1. 00 gram of a solid, 5. If the solid was Na. HCO 3, calculate how many moles reacted. 1. 00 g Na. HCO 3 ÷ 84. 007 g Na. HCO 3 = 0. 0119 mol Na. HCO 3 6. If the solid was Na 2 CO 3, calculate how many moles reacted. 1. 00 g Na 2 CO 3 ÷ 105. 988 g Na 2 CO 3 = 0. 009435 mol Na CO 2 3

Using your answers from 5 & 6 and the balanced chemical equations, 7. If the unknown was Na. HCO 3, how many moles of Na. Cl would have been produced? 8. If the unknown was Na 2 CO 3, how many moles of Na. Cl would have been produced? 9. Identify the unknown as Na. HCO 3 or Na 2 CO 3. Explain how you know. Results -
- Slides: 5