Lab 7 Diffusion and Osmosis Objectives Understand what
Lab 7: Diffusion and Osmosis Objectives § Understand what is Brownian Movement § Understand that the principle of osmosis and diffusion in animal and plant physiological systems. § Predict the net movement of molecules across a semipermeable membrane. § Define what molecules move and their relative size during diffusion and osmosis.
Overview –Thermal molecular motion Brownian Movement is random motion of particles of a matter in a liquid (random thermal motion). Liquid molecules themselves (such as water molecules) also subject to random thermal motion. Factors affect random thermal motion: 1) Temperature and molecular motion: § For example, water molecules in hot water move faster than those of cold water. § For example, water molecules in water vapor (gas) move faster than those in liquid water. 2) Molecular weight and molecular motion A substance with low molecular weight move faster than those with higher molecular weight
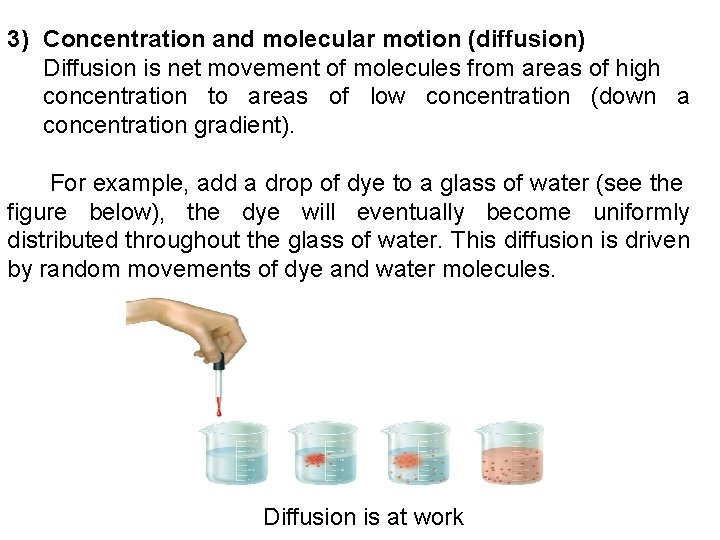
3) Concentration and molecular motion (diffusion) Diffusion is net movement of molecules from areas of high concentration to areas of low concentration (down a concentration gradient). For example, add a drop of dye to a glass of water (see the figure below), the dye will eventually become uniformly distributed throughout the glass of water. This diffusion is driven by random movements of dye and water molecules. Diffusion is at work
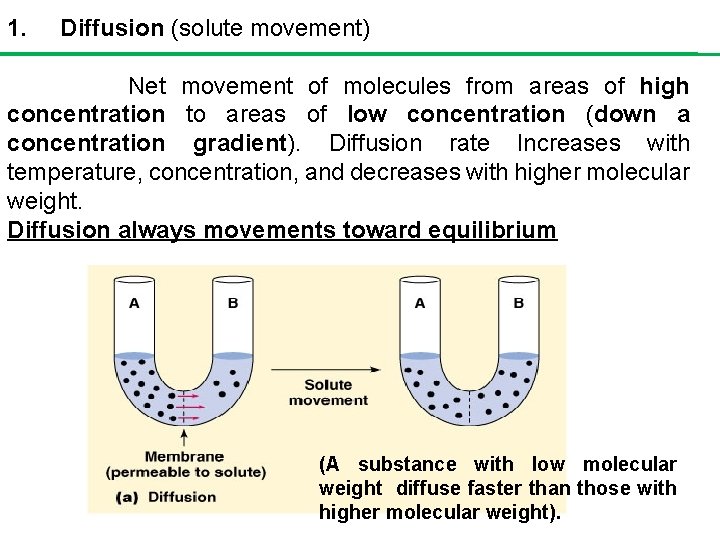
1. Diffusion (solute movement) Net movement of molecules from areas of high concentration to areas of low concentration (down a concentration gradient). Diffusion rate Increases with temperature, concentration, and decreases with higher molecular weight. Diffusion always movements toward equilibrium (A substance with low molecular weight diffuse faster than those with higher molecular weight).
2. Diffusion and Differentially Permeable Membranes (1) What are hydrophilic, hydrophobic, polar , and nonpolar molecules? § § § Hydrophilic: Solutes that have an affinity for water and therefore dissolve readily in it are called hydrophilic. Most small organic molecules found in cells are hydrophilic, such as sugars, organic acids, and some of the amino acids. Hydrophobic: Molecules that are not very soluble in water are termed hydrophobic, such as lipid. Polar molecules: have positively or negatively charged regions. Nonpolar Molecules: have no local regions of positively or negatively charge. In general, polar molecules tend to be hydrophilic and nonpolar molecules tend to be hydrophobic.

(2) Cell membrane is phospholipid bilayer with proteins embedded in (Fluid mosaic model of biological membranes) § Phospholipid bilayer membranes form hydrophobic region that are permeable to hydrophobic molecules (lipids such as steroid hormones, fatty acids, polymers of fatty acids, etc. ) and small non-polar molecules (gases such as O 2, H 2, N 2, CO 2, He, etc. ). § Both heads of phospholipid bilayer membranes are hydrophilic and are impermeable to hydrophilic or polar molecules (poorly permeable to water and practically impermeable to larger hydrophilic molecules such as sugars, amino acids, nucleotides, and their polymers) and impermeable to ions, no matter how small (e. g. H+, OH-, Na+, Cl-).

§ Some of membrane proteins contain pores or channels to allow polar and ionic molecules to cross the plasma membrane. Channel proteins and carrier proteins allow the transport or diffusion of ions or polar molecules into or out of the cell. § Dialysis tubing consists of a selectively permeable chemical membrane, and can serve as a model to explore some of the properties of biological membranes: submicroscopic pores (holes) in cellulose membrane lets smaller molecules (water, small ions, amino acids, monosaccharides) through easily, but is impermeable to larger ones (polysaccharides, proteins).
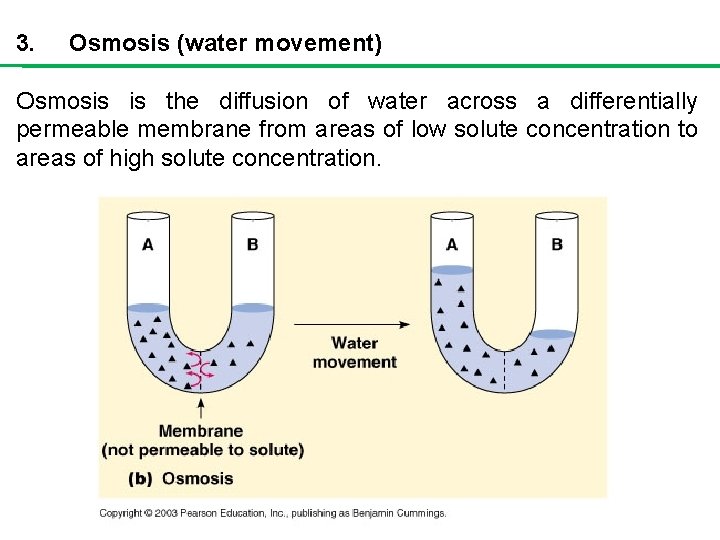
3. Osmosis (water movement) Osmosis is the diffusion of water across a differentially permeable membrane from areas of low solute concentration to areas of high solute concentration.
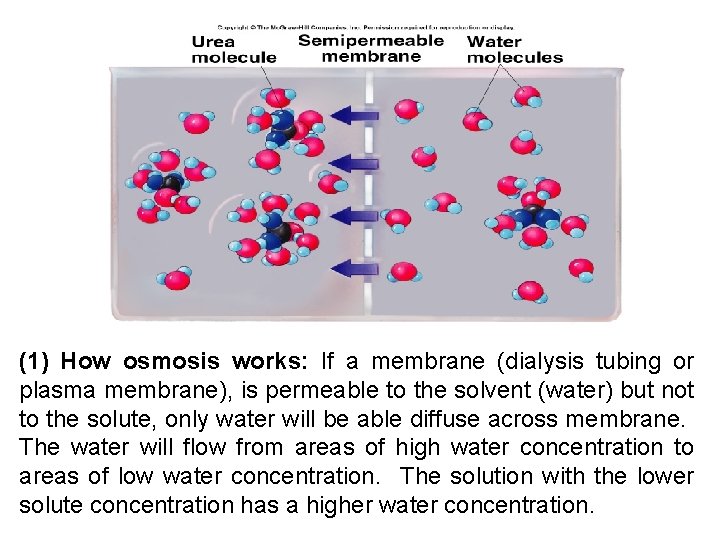
(1) How osmosis works: If a membrane (dialysis tubing or plasma membrane), is permeable to the solvent (water) but not to the solute, only water will be able diffuse across membrane. The water will flow from areas of high water concentration to areas of low water concentration. The solution with the lower solute concentration has a higher water concentration.

(2) Isotonic, hypertonic, and hypotonic solutions. Hypertonic: The solution with the higher solute concentration is called hypertonic. Hypotonic: The solution with the lower solute concentration is called hypotonic. Isotonic: If two solutions have the same solute concentration, they are said to be isotonic. During osmosis, there are is a net water movement from the hypotonic solution towards the hypertonic solution. For isotonic solutions, no osmosis will occur.
4. § § § Osmosis in Animal Cells (red blood cells): Red blood cells in a isotonic solution: Animal cells live in a isotonic environment: the cytoplasm of red blood cells is isotonic with blood or tissue fluid. No osmosis will occur and red blood cells will have their normal shape, a biconcave disk. Red blood cells in a hypotonic solution: Water will flow into cells and create a pressure inside the cell (hydrostatic pressure) as the cell swells. A high hydrostatic pressure may break the plasma membrane, to burst the cell open, a process called cell lysis. Red blood cells in a hypertonic solution: Water will flow out of cells, shriveling the cells. The cell will not complete deflate, as the cytoskeleton prevents the complete collapse of the membrane on itself. Red blood cells will show ridged edges and are said to be in the crenate shape.

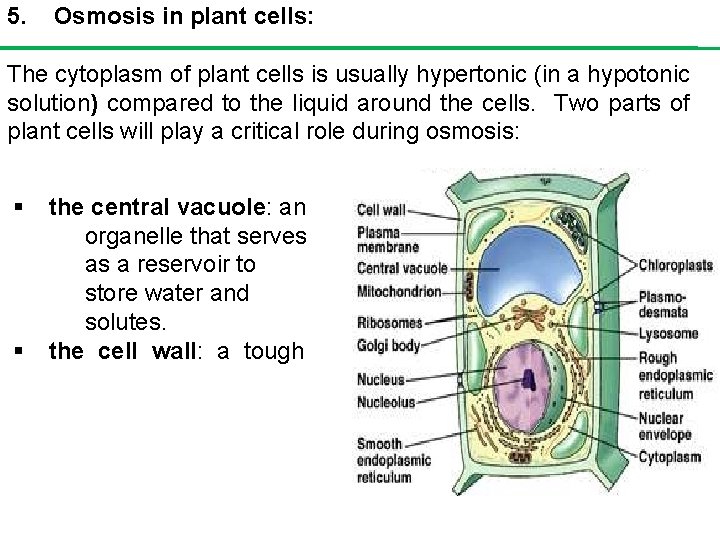
5. Osmosis in plant cells: The cytoplasm of plant cells is usually hypertonic (in a hypotonic solution) compared to the liquid around the cells. Two parts of plant cells will play a critical role during osmosis: § § the central vacuole: an organelle that serves as a reservoir to store water and solutes. the cell wall: a tough
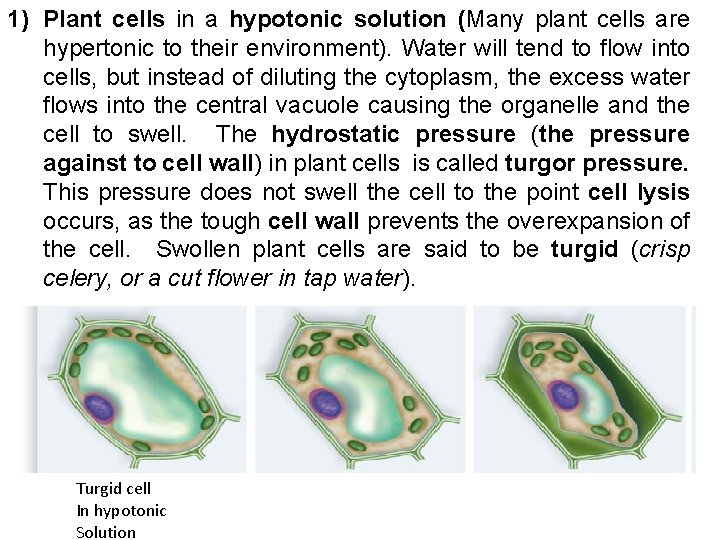
1) Plant cells in a hypotonic solution (Many plant cells are hypertonic to their environment). Water will tend to flow into cells, but instead of diluting the cytoplasm, the excess water flows into the central vacuole causing the organelle and the cell to swell. The hydrostatic pressure (the pressure against to cell wall) in plant cells is called turgor pressure. This pressure does not swell the cell to the point cell lysis occurs, as the tough cell wall prevents the overexpansion of the cell. Swollen plant cells are said to be turgid (crisp celery, or a cut flower in tap water). Turgid cell In hypotonic Solution
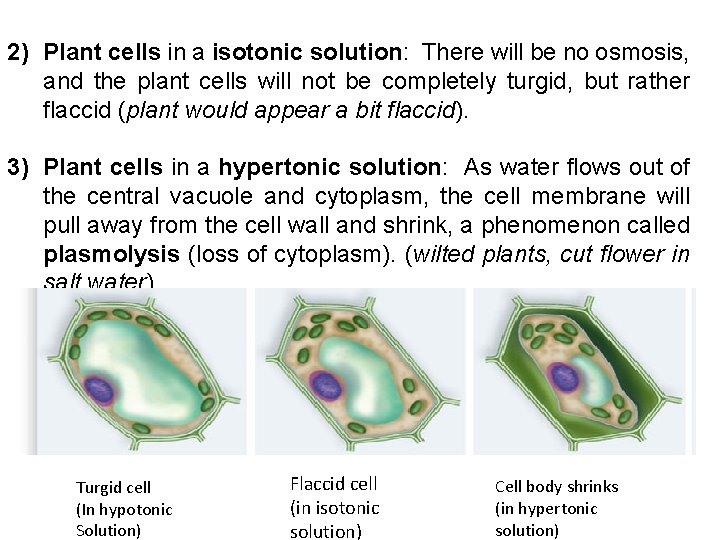
2) Plant cells in a isotonic solution: There will be no osmosis, and the plant cells will not be completely turgid, but rather flaccid (plant would appear a bit flaccid). 3) Plant cells in a hypertonic solution: As water flows out of the central vacuole and cytoplasm, the cell membrane will pull away from the cell wall and shrink, a phenomenon called plasmolysis (loss of cytoplasm). (wilted plants, cut flower in salt water). Turgid cell (In hypotonic Solution) Flaccid cell (in isotonic solution) Cell body shrinks (in hypertonic solution)
- Slides: 15