Lab 6 Immunohistochemistry I H C Prepared by

Lab #6: Immunohistochemistry ( I H C) Prepared by: El-Hindi. M. & Abdelmoneim. A. Practical Of Histopathology 2015 1
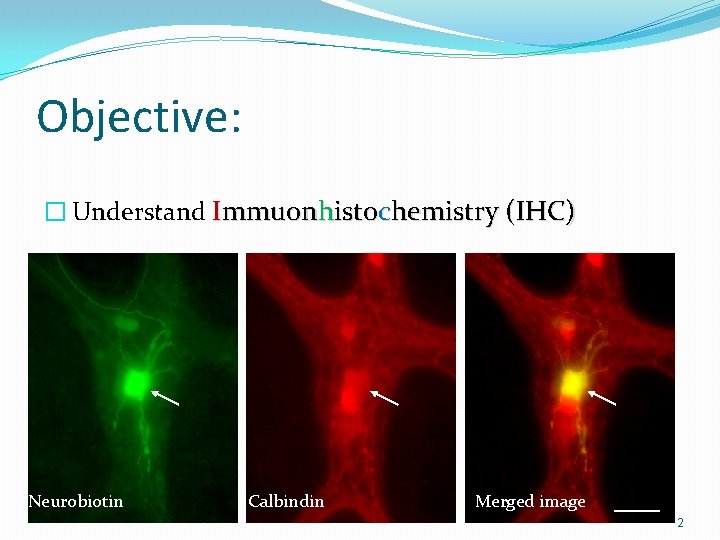
Objective: � Understand Immuonhistochemistry (IHC) Neurobiotin Calbindin Merged image 2

Overview �Imunohistochemistry (IHC) combines histological, histological immunological and biochemicaltechniques for the identification of specific tissue components by means of a specific antigen/antibody reaction tagged with a visible label 3
Cont. � The body's response to the introduction of a foreign agent, known as the immune response, response results in the production of antibodies which bind the offending material. �Antibodies bind tightly and specifically to an "epitope" epitope (one specific structure) on an "antigen" antigen (foreign molecule or structure). 4
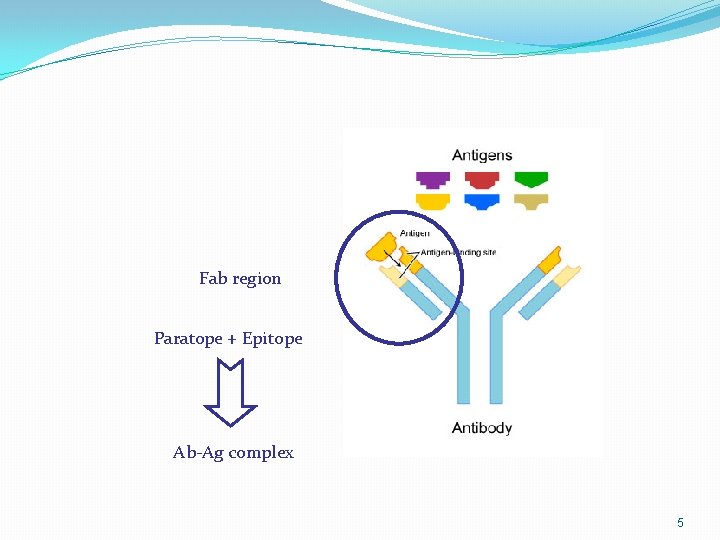
Fab region Paratope + Epitope Ab-Ag complex 5

6
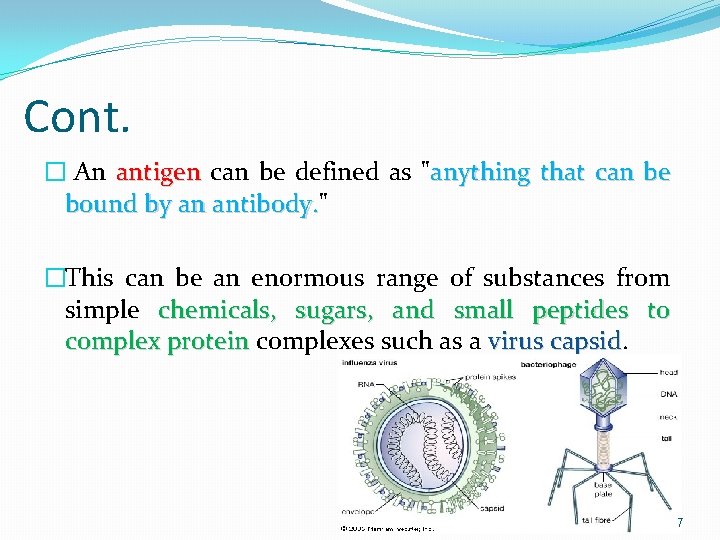
Cont. � An antigen can be defined as "anything that can be bound by an antibody. " antibody. �This can be an enormous range of substances from simple chemicals, sugars, and small peptides to complex protein complexes such as a virus capsid 7
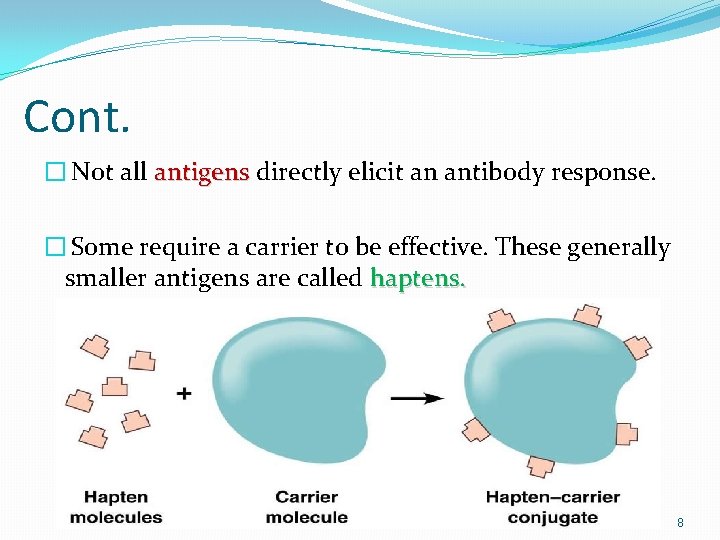
Cont. � Not all antigens directly elicit an antibody response. � Some require a carrier to be effective. These generally smaller antigens are called haptens. 8
Cont. � Antibodies can be generated by injecting animals with antigens, antigens and then collecting serum after the immune response has taken place. �If the antibodies are labeled with an easily detectable molecule (a fluorescent dye, an enzyme, etc. ), etc they become powerful detection reagents for the antigen �This system has been exploited to generate exceptionally specific and sensitive "stains" which are used in histology as well as other disciplines. 9
Cont. � The basic process depends upon selecting an antibody sufficiently specific to bind an antigen in situ � The antibody/antigen conjugate is then identified using a variety of signal generating molecules triggered either by the antibody/antigen interaction or by secondary processes �The signal generators can be precipitating dyes, fluorescent molecules or electron dense (ultrastructural tag) materials for electron microscopy (EM) 10
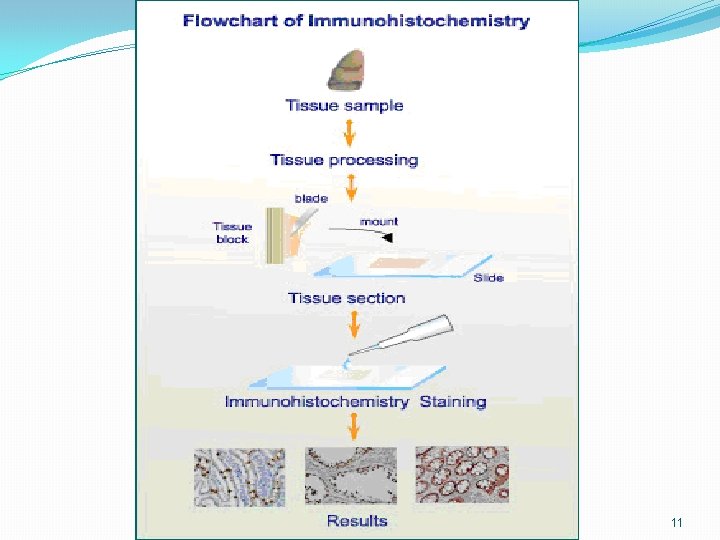
11

Cont. � Immunohistochemistry is generally carried out in sectioned tissue, which allows the antibodies free access to the interior of the cells � Immunohistochemistry can also be carried out on cells either in free solution or bound to membranes, or on monolayers of cultured cells 12

Cont. � Intracellular Immunohistochemistry requires that the antibody to the target antigen be able to penetrate the cell membrane and whatever cell wall may be present before it can attach to the antigen. �This requires a number of steps not required for sectioned tissue. 13
Cont. � Primarily the cell membrane must be made permeable to the antibody, antibody though at the same time the integrity of the cell contents and structures must be maintained. �This is normally achieved through the use of a specialized buffer containing a detergent 14
15
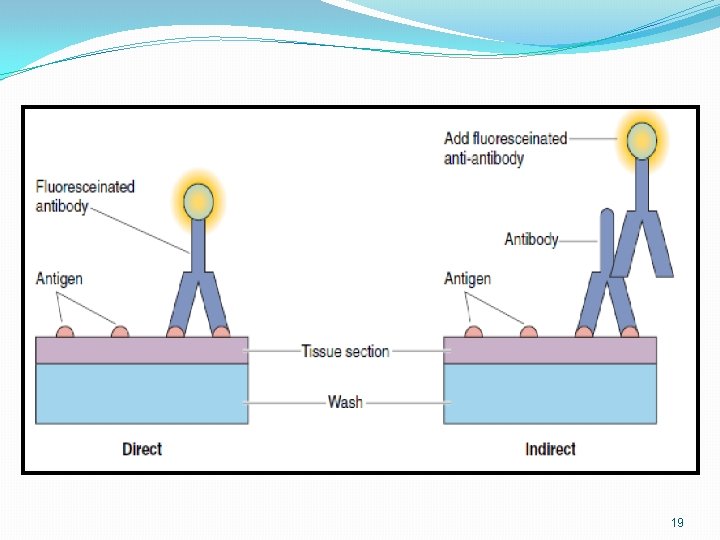
Direct Indirect Methods of antibody labeling 16
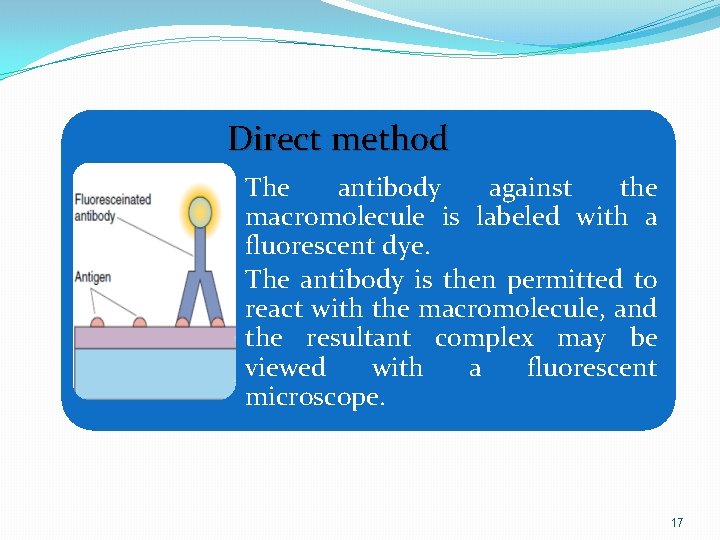
Direct method • The antibody against the macromolecule is labeled with a fluorescent dye. • The antibody is then permitted to react with the macromolecule, and the resultant complex may be viewed with a fluorescent microscope. 17
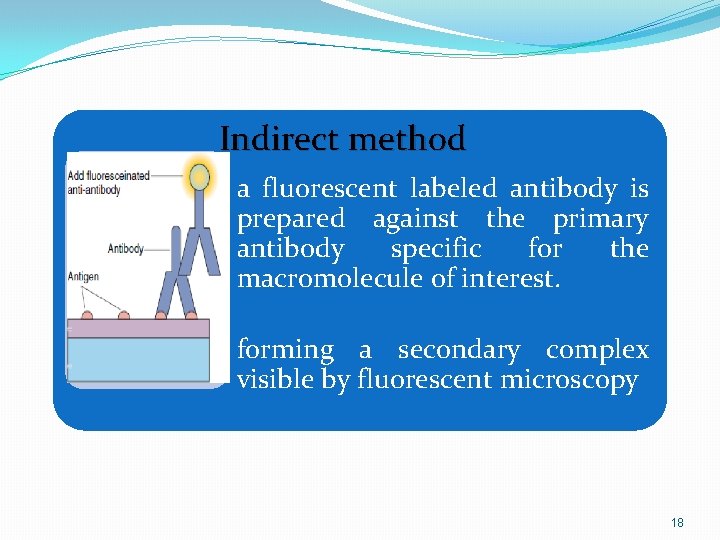
Indirect method • a fluorescent labeled antibody is prepared against the primary antibody specific for the macromolecule of interest. • forming a secondary complex visible by fluorescent microscopy 18
19

Cont. �The indirect method is more sensitive than the direct method because numerous labeled anti-antibodies bind to the primary antibody, making them easier to visualize. �In addition, the indirect method does not require labeling of the primary antibody, antibody which often is available only in limited quantities 20

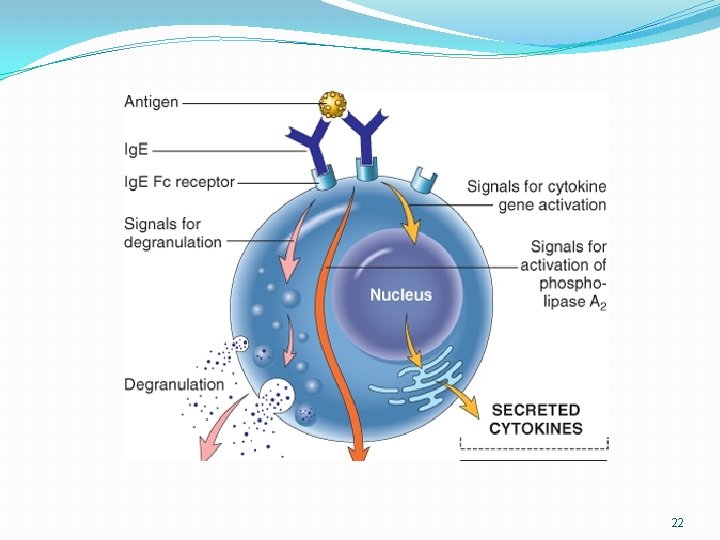
Cont. �Immunocytochemistry can be used with specimens for electron microscopy by labeling the antibody with ferritin, an electron-dense molecule, instead of with a fluorescent dye �Ferritin labeling can be applied in both the direct and indirect methods. 21
22

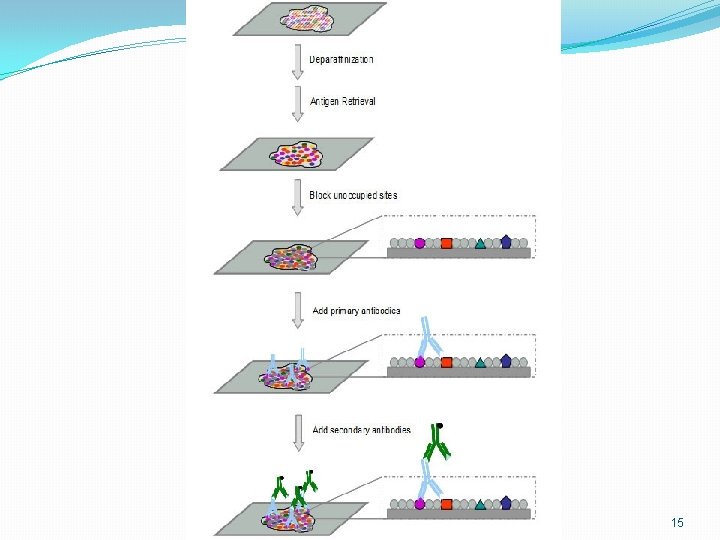
General Immunohistochemistry Protocol 23

Part 1 Tissue preparation 1. Fixation Fresh unfixed, or formalin fixation and paraffin embedding 2. Sectioning 3. Whole Mount Preparation 24
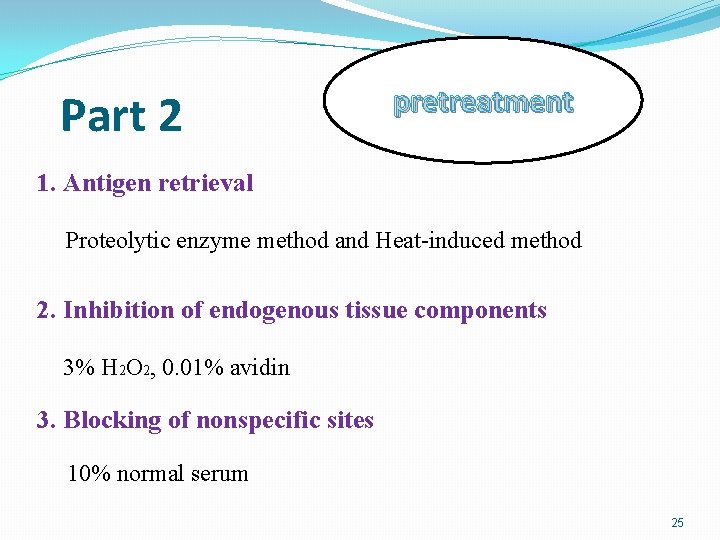
Part 2 pretreatment 1. Antigen retrieval Proteolytic enzyme method and Heat-induced method 2. Inhibition of endogenous tissue components 3% H 2 O 2, 0. 01% avidin 3. Blocking of nonspecific sites 10% normal serum 25

Part 3 staining �Make a selection based on the type of specimen, the primary antibody, the degree of sensitivity and the processing time required. 26

Controls �Positive Control It is to test for a protocol or procedure used. It will be ideal to use the tissue of known positive as a control. �Negative Control It is to test for the specificity of the antibody involved. 27
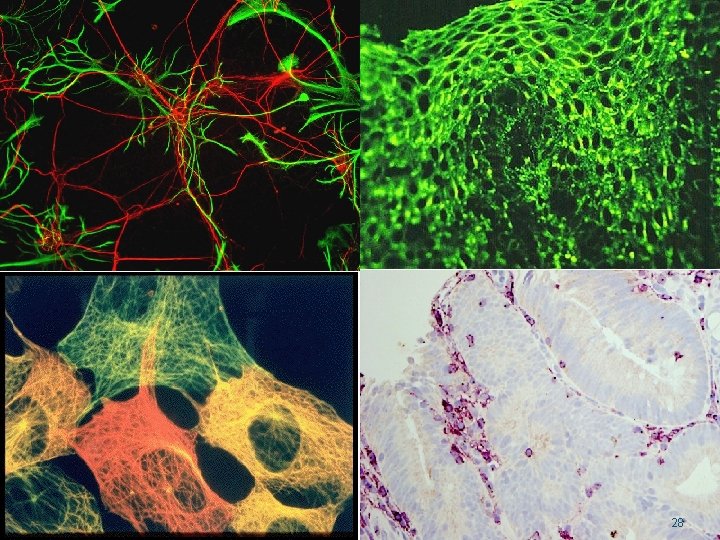
28
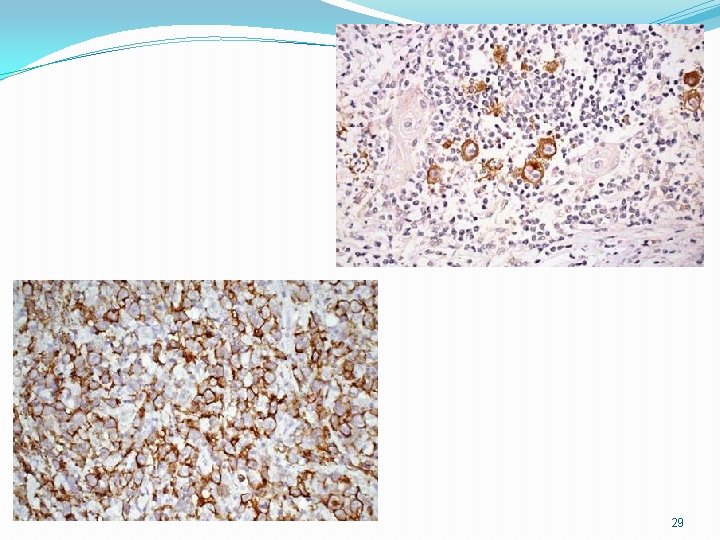
29
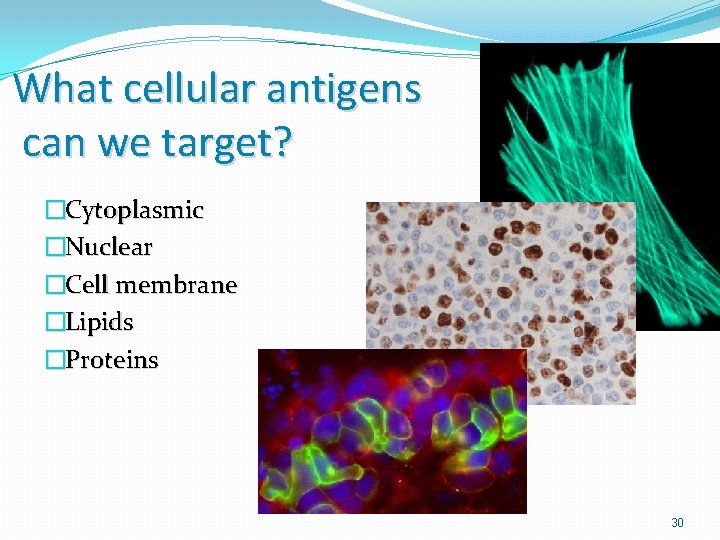
What cellular antigens can we target? �Cytoplasmic �Nuclear �Cell membrane �Lipids �Proteins 30
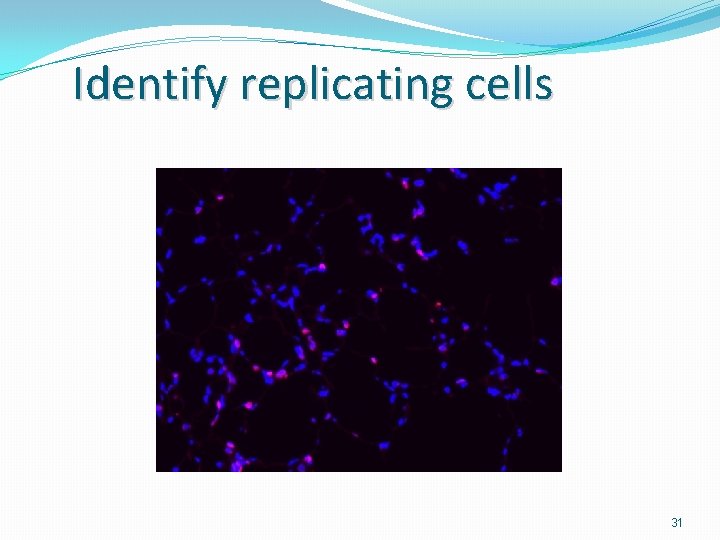
Identify replicating cells 31
Locate cells that are signaling 32
Locate apoptotic cells 33
Identify activation states 34
Identify different types of cells in a tissue 35
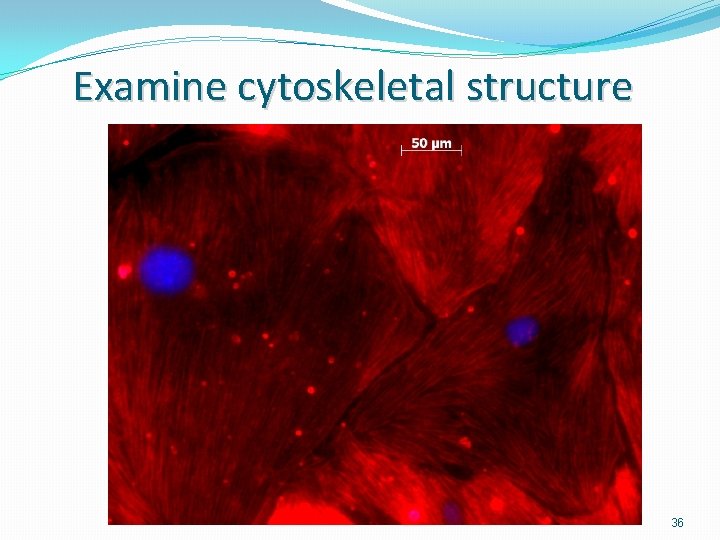
Examine cytoskeletal structure 36

37
- Slides: 37