Lab 5 Cell Respiration equation C 6 H
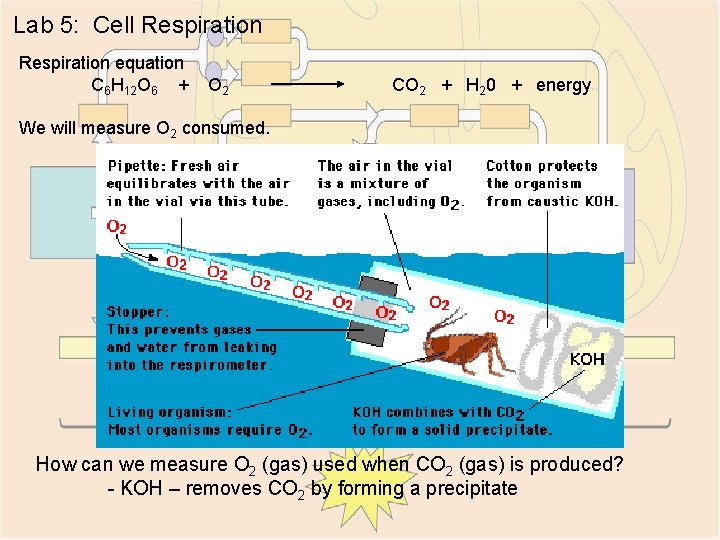
Lab 5: Cell Respiration equation C 6 H 12 O 6 + O 2 CO 2 + H 20 + energy We will measure O 2 consumed. How can we measure O 2 (gas) used when CO 2 (gas) is produced? - KOH – removes CO 2 by forming a precipitate
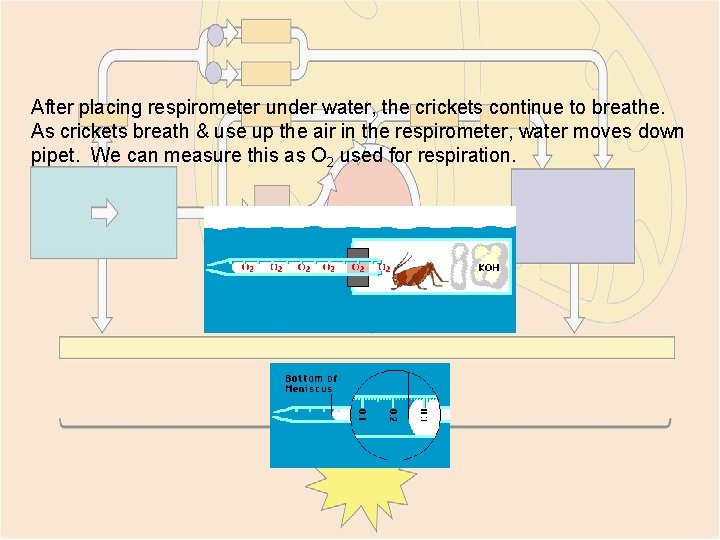
After placing respirometer under water, the crickets continue to breathe. As crickets breath & use up the air in the respirometer, water moves down pipet. We can measure this as O 2 used for respiration.
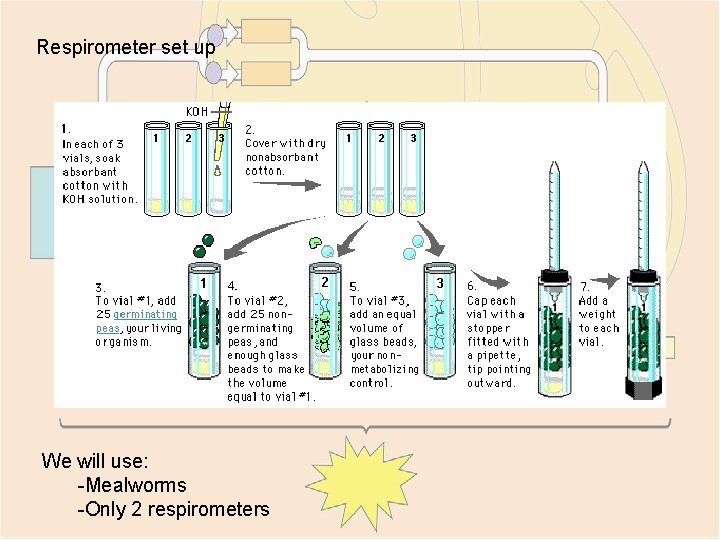
Respirometer set up We will use: -Mealworms -Only 2 respirometers
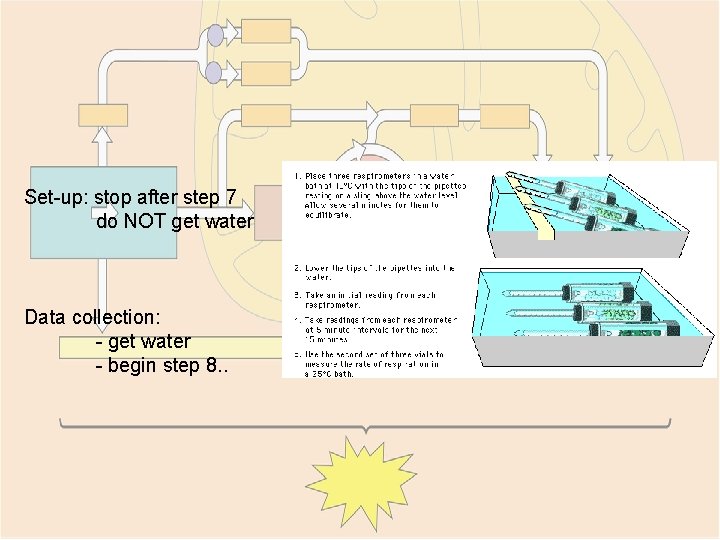
Set-up: stop after step 7 do NOT get water Data collection: - get water - begin step 8. .
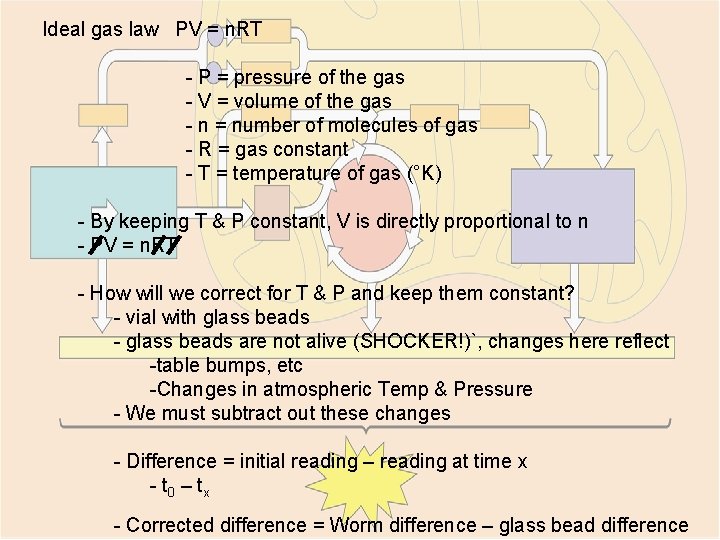
Ideal gas law PV = n. RT - P = pressure of the gas - V = volume of the gas - n = number of molecules of gas - R = gas constant - T = temperature of gas (°K) - By keeping T & P constant, V is directly proportional to n - PV = n. RT - How will we correct for T & P and keep them constant? - vial with glass beads - glass beads are not alive (SHOCKER!)`, changes here reflect -table bumps, etc -Changes in atmospheric Temp & Pressure - We must subtract out these changes - Difference = initial reading – reading at time x - t 0 – t x - Corrected difference = Worm difference – glass bead difference

Lab Notebook Format • • 1) Title/Date 2) Pre-Lab (Key Concepts & Materials) 3) Purposes (2 -3) 4) Personal Account – Hypothesis, Procedure (all steps), Data Table, Graph, Reaction Rate Calculations • 5) Discussion Questions (#1 -8) – Exclude #1, #3, #5—these should already be present in your lab entry • 6) Conclusion
- Slides: 6