Lab 41 Laboratory analysisaccuracy and precision Objectives O

Lab 41 Laboratory analysis—accuracy and precision Objectives: O 1: Significant figures and precision O 2: Discrepancy formula and accuracy O 3: Rulers and protractors 1

The precision of a measurement is given by its number of significant figures. (Note these guidelines on page 5. ) 1) Count the number of digits: 2355 = 4 significant figures 124. 302 = 6 significant figures 2) Don’t count “following zeros”: 2300 = 2 significant figures 10000 = 1 significant figure 3) Don’t count “leading zeros”: 0. 0033 = 2 significant figures 4) But…count “following zeros” if they’re made significant with a decimal: 2300. = 4 significant figures 100000. = 6 significant figures 5) And…count “following zeros” in numbers less than 1 0. 3300 = 4 significant figures 6) The exponents in scientific notation do not contribute to sig figs: 6. 32 x 1011 = 3 significant figures 7) Finally, numbers and constants in equations and conversions have unlimited precision: Area = 4 π R 2

Significant figures in calculations: 1) Plug your numbers into your calculator as normal. 2) If you have to stop at some intermediate step, record your answer with excessive significant figures. 3) When you are completely finished, round your value to the same number of significant figures as the significant figures in your LEAST PRECISE input number. Example: A cylinder has a diameter of 14. 233 cm, and a height of 18 cm. What is its volume? Volume = (π/4) x D 2 x H = (3. 1416/4) x (14. 233 cm)2 x 18 cm = 2863. 88 cm 3 = 2900 cm 3

One final obstacle: When the result of a calculation is hard to write properly… Example: You measure, with great accuracy, the sides of a rectangular block to be 15. 8 mm, 20. 1 mm, and 12. 6 mm. When you calculate the volume, you get Volume = L x W x H = 15. 8 mm x 20. 1 mm x 12. 6 mm = 4002 mm 3 How do you write “ 4002 mm 3” to three significant figures? 4002 mm 3 has 4 significant figures, while 4000 mm 3 has only 1! Solution: you must provide your answer in scientific notation: V = 4. 00 x 103 mm 3

Discrepancy and accuracy: If there are two estimates for the same value, you can say how much they differ from each other, by the discrepancy formula. It will take us FIVE HOURS! Why don’t we just examine these claims using the discrepancy formula? ? ? Are you crazy? ? It will take us SIX HOURS!
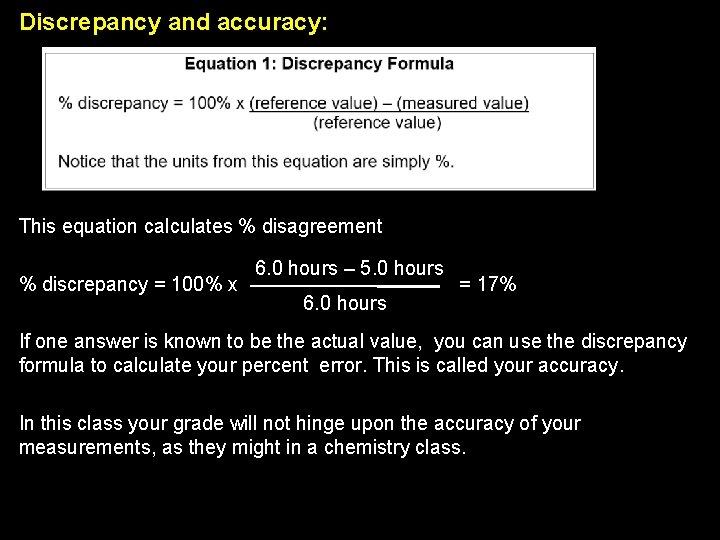
Discrepancy and accuracy: This equation calculates % disagreement % discrepancy = 100% x 6. 0 hours – 5. 0 hours 6. 0 hours = 17% If one answer is known to be the actual value, you can use the discrepancy formula to calculate your percent error. This is called your accuracy. In this class your grade will not hinge upon the accuracy of your measurements, as they might in a chemistry class.

Protractors 33º, NOT 147º Accuracy of ± 1° is acceptable. Don’t be sloppy.
Protractors
- Slides: 8