Lab 4 Some Purification Technique for Solids 1

Lab 4

Some Purification Technique for Solids 1 -Recrystallization. 2 -Extraction. 3 -Distillation. 4 -Chromatography.

Introduction Recrystallization The separation of a solid mixture. Purification by recrystallization depends on the following facts: 1 -Different solids have different solubilities in a given solvent. 2 -Most solids are more soluble in hot than in cold solvents
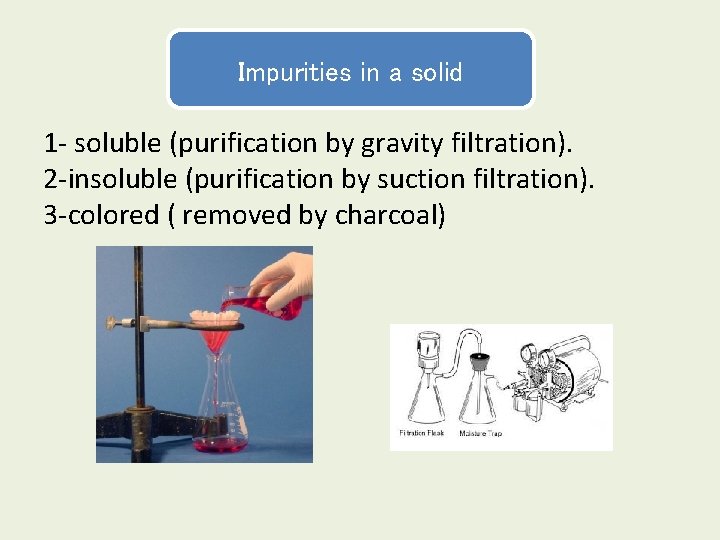
Impurities in a solid 1 - soluble (purification by gravity filtration). 2 -insoluble (purification by suction filtration). 3 -colored ( removed by charcoal)

The solubility of a solid solute in a solvent is determined by two factors: 1 -"Like dissolves like". 2 -The lattice energy of the crystalline solute.

A suitable solvent for recrystallization should possess the following important properties: 1 -Dissolve a large amount of the solid to be purified at high temperatures, but very little at room temperature. 2 -Dissolve impurities readily at low temperatures or not at all even at the boiling point. 3 -Not react with the substance to be purified. 4 -Evaporate readily from the crystals, i. e. , be relatively volatile.
If two or more solvents appear to be equally suitable, it is preferable to choose a solvent which is non-flammable non-toxic and cheap.
Recrystallization involves the following sequence of steps: 1 -Selection of a suitable solvent. 2 -Preparation of the hot solution and "decolorization" if necessary. 3 -Filtration of the hot solution to remove insoluble impurities (and charcoal). 4 -Cooling slow then using ice (5 -10 min) 5 -Collection (cold filtration) by suction filtration. 6 - Washing(three times) and drying of the crystals.

Objectives 1 -Selection of suitable solvents for recrystallization. 2 -Recrystallization of an unknown compound.
Experimental A-Selection of solvent: Perform solubility tests on anthracene, salicylic acid, and sodium benzoate in water, alcohol, and ligroin as follows: 1 -With a spatula take about 0. 1 g of the powdered solid and place in a dry test tube. 2 -Start by dissolving it in 2 m. L of solvent with stirring. If insoluble, heat the mixture to boiling (water bath for flammable solvents) and observe the solubility.

B-Recrystallization of an unknown compound 1 -Selection of suitable solvents. 2 -Recrystallize 1. 0 g of this unknown from the solvent you have selected. (Make sure you use only the minimum volume of solvent).
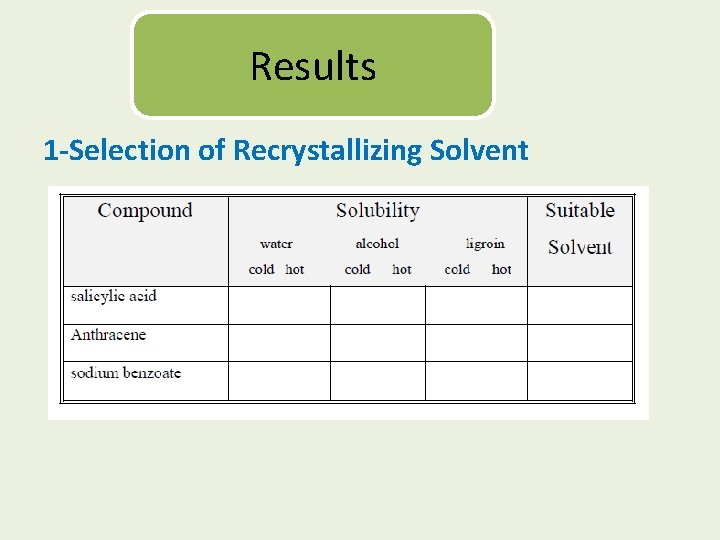
Results 1 -Selection of Recrystallizing Solvent
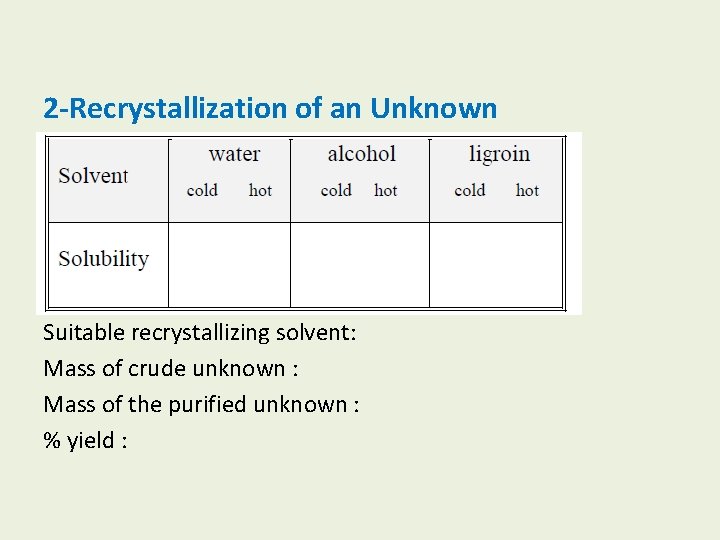
2 -Recrystallization of an Unknown Suitable recrystallizing solvent: Mass of crude unknown : Mass of the purified unknown : % yield :
- Slides: 13