Lab 4 Osmotic pressure The Osmolar concentration mainly

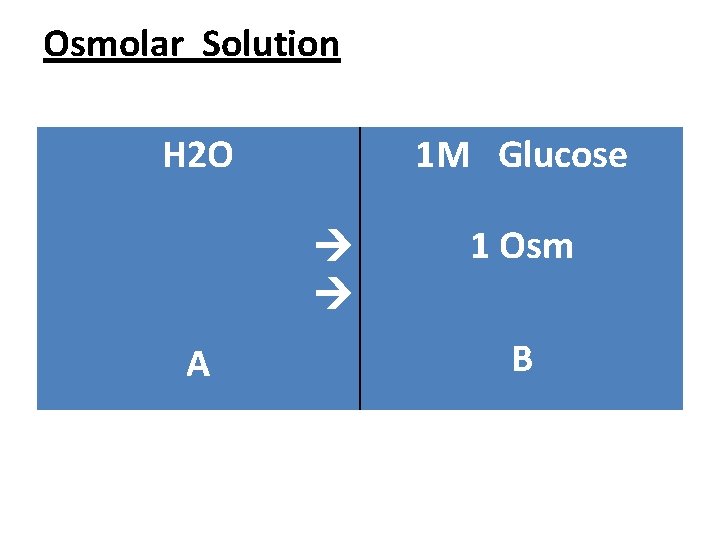
Lab 4 Osmotic pressure The Osmolar concentration mainly used in biology to explain the osmotic effect, We suggest the existence of a container divided by water permeable membrane to two sides A and B. water presence in both sides and glucose (1 M) in side B only

Osmolar Solution • So to reaching equilibrate stage the water will move from (A to B) { from high water concentration to low water concentration }. This movement of water called Osmosis , and the power that mated on the membrane called Osmotic pressure
Osmolar Solution H 2 O 1 M Glucose A 1 Osm B

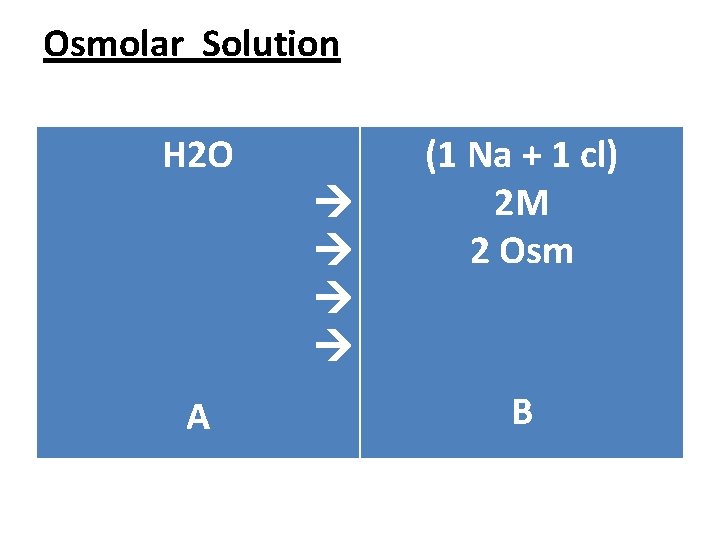
Osmolar Solution • ● Osmol (Osm) : A unite used to explain the capability of a solution to make Osmotic pressure. • ◙ Every (1 m) of glucose sheds osmotic pressure on membrane about (1 Osm) this applies to all non ionic molecules. So if we put (2 M) of glucose in the side B this will cause ↑ in water movement from A side to B side causing ↑ in Osmotic pressure.
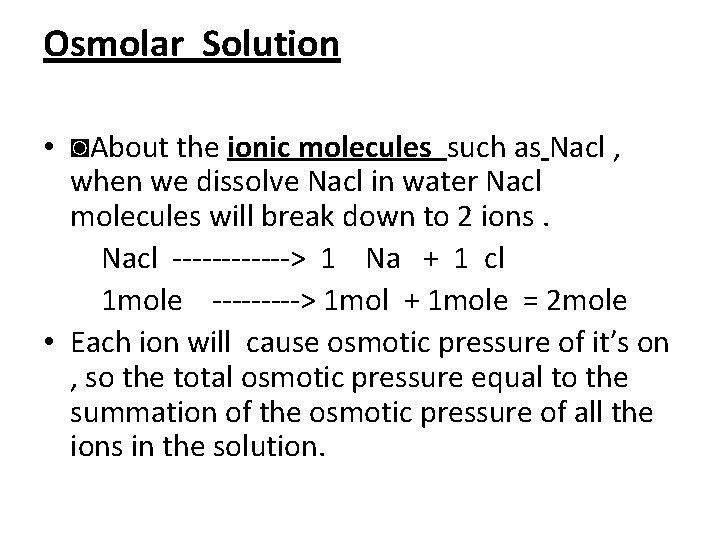
Osmolar Solution • ◙About the ionic molecules such as Nacl , when we dissolve Nacl in water Nacl molecules will break down to 2 ions. Nacl > 1 Na + 1 cl 1 mole > 1 mol + 1 mole = 2 mole • Each ion will cause osmotic pressure of it′s on , so the total osmotic pressure equal to the summation of the osmotic pressure of all the ions in the solution.
Osmolar Solution H 2 O A (1 Na + 1 cl) 2 M 2 Osm B

Osmolar Solution • ◙ Osmotic pressure of all mammals cell is 0. 3 Osm {300 m. Osm} milliosmolar , if we put normal cell in solution of • 0. 3 Osm of glucose. • Or 0. 15 Osm of Nacl • This cell will be intact , this solution called isotonic solution.

Osmolar Solution I. Isotonic Solution : A solution with osmotic pressure equal to osmotic pressure inside mammals’ cells. {the cell remain intact} II. Hypertonic Solution : A solution with osmotic pressure higher than the osmotic pressure inside the cell. {the cell will be Shrinks } III. Hypotonic Solution : A solution with osmotic pressure lower than the osmotic pressure inside the cell. {the cell will be destroyed }
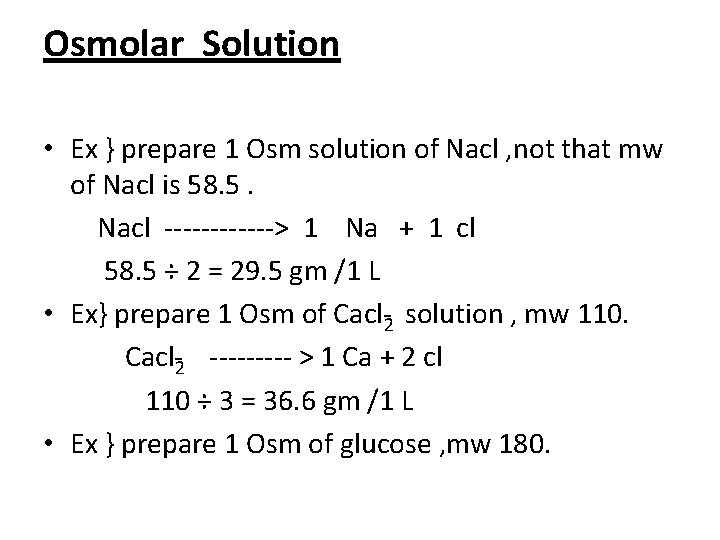
Osmolar Solution • Ex } prepare 1 Osm solution of Nacl , not that mw of Nacl is 58. 5. Nacl > 1 Na + 1 cl 58. 5 ÷ 2 = 29. 5 gm /1 L • Ex} prepare 1 Osm of Cacl 2 solution , mw 110. Cacl 2 > 1 Ca + 2 cl 110 ÷ 3 = 36. 6 gm /1 L • Ex } prepare 1 Osm of glucose , mw 180.
- Slides: 9