Lab 4 6 Methanol as a Solvent Due
Lab 4. 6: Methanol as a Solvent Due: Wed. Feb. 12, 2014

Question Citric acid and sugar look the same, they have about the same density, and they are both soluble in water. How can we distinguish the two based on solubility?

Methanol as a Solvent Purpose: To compare solubility using water and methanol as solvents Hypothesis: If I use water and methanol as solvents, then… because… Materials: (2) test tubes, (2) stoppers, sugar, citric acid, methanol, scoopula, graduated cylinder

Procedure 1. Fill both test tubes with 5 ml of methanol 2. Use tip of scoopula to add citric acid into one test tube. Repeat this for sugar in the other test tube. 3. Stopper the test tubes and shake. 4. Repeat steps 2 & 3 until solution becomes saturated. 5. Keep track of how many times you add each solute into the test tube. 6. Make observations. 7. Clean up. POUR SOLUTIONS IN WATER JUG AT THE BACK SINK!!!
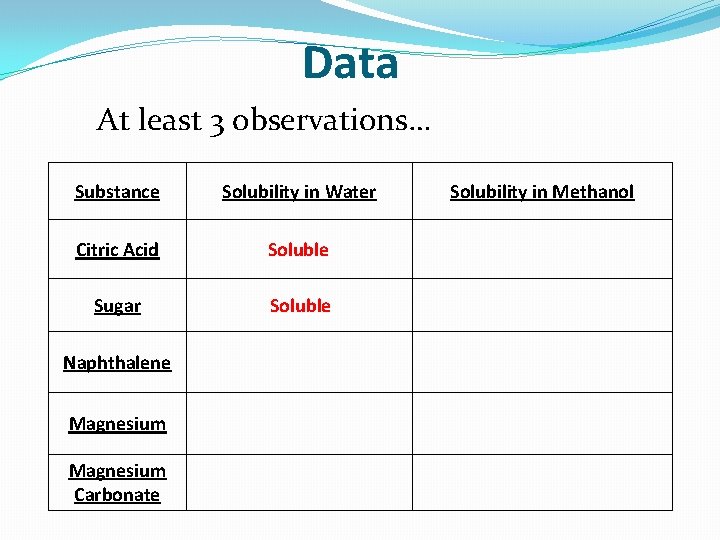
Data At least 3 observations… Substance Solubility in Water Citric Acid Soluble Sugar Soluble Naphthalene Magnesium Carbonate Solubility in Methanol

Research Questions & Answers Questions 15 & 16 from lab manual

Conclusion To compare the solubility using water and methanol and solvents, I found that… �Was methanol an appropriate solvent in helping to distinguish citric acid and sugar? Use data table as support. �Were you able to distinguish naphthalene, magnesium, and magnesium carbonate using methanol and water as solvents? Use data table support. �If you are trying to distinguish between different solids that look the same, how can you accomplish this using what you know from this lab? � Agree/disagree with hypothesis � Real world example � Theory in science this lab relates to � What you learned
- Slides: 7