Lab 3 Building a Spectroscope Fall 2018 Chemistry

Lab 3: Building a Spectroscope Fall 2018 Chemistry 1331: Chemical Structures and Properties Tues. Sept. 18 - Mon. Sept. 24

Background • • Electromagnetic Spectrum E = hn c = ln Absorption and Emission Parts of the EM spectrum / Chemical event

Pre-Lab • Any Questions? • Take 5/10 minutes to take the pre-lab
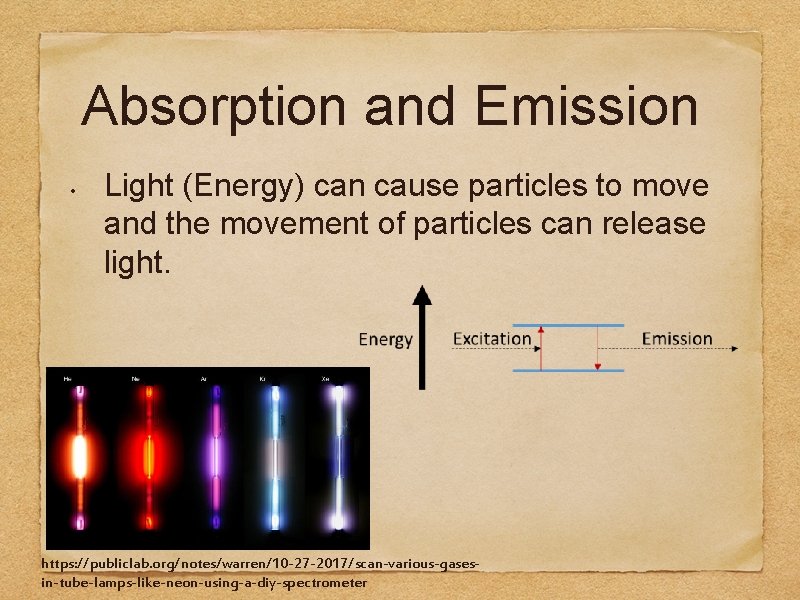
Absorption and Emission • Light (Energy) can cause particles to move and the movement of particles can release light. https: //publiclab. org/notes/warren/10 -27 -2017/scan-various-gasesin-tube-lamps-like-neon-using-a-diy-spectrometer

Building a Spectrometer • A Spectrometer basically consist of 3 parts: • Light Source • Grating – separates the light into component wavelengths • Detector Kkmurray. Spectrometer Schematic. https: //commons. wikimedia. org/wiki/File: Spectrometer_schematic. gif (Accessed 9/12/2018)

Procedure to Build • • Cut along the solid lines of the supplied design. Fold along the dotted lines. Using the black paper build a very small slit between two small pieces of paper. Cut out small pieces of black paper and tape them to the inside of the spectrometer. Using a DVD, prepare a small piece of diffraction grating.

Using a Spectrometer • • Aligning the spectrometer is critical. • It might take some time. If you get it aligned with your camera, tape it in place. https: //publiclab. org/wiki/spectral-workbench-calibration

Procedure • • This will take some time. Aligning it correctly is not instantaneous, but you have plenty of time. Calibrate • • • Calibrate using a CFL Lamp Test the Spectrometer on a Neon Emission Tube. Test the Spectrometer on Air, Oxygen, and Nitrogen.

Next Week • For Next week. • Read Lab #4, be prepared to discuss.
- Slides: 9