Lab 15 Date Chemical Reactions I Purpose Observe
Lab 15 Date: Chemical Reactions I Purpose Observe and classify different types of chemical reactions
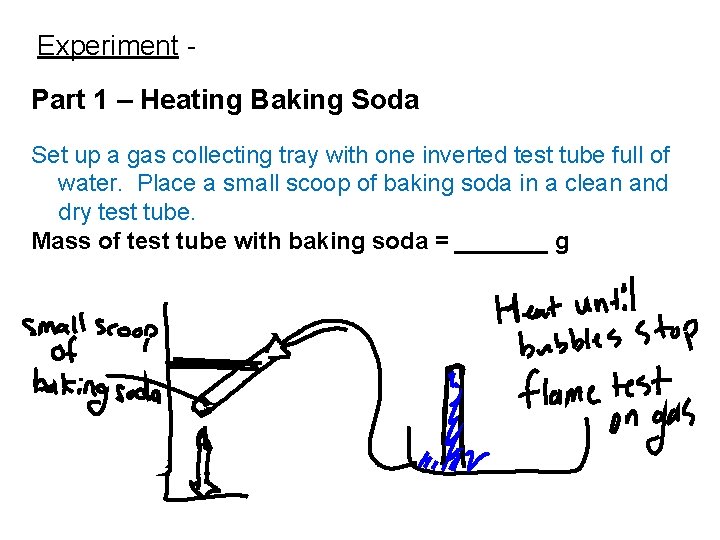
Experiment Part 1 – Heating Baking Soda Set up a gas collecting tray with one inverted test tube full of water. Place a small scoop of baking soda in a clean and dry test tube. Mass of test tube with baking soda = _______ g

Begin heating the test tube and collecting the gas in the inverted test tube produced from the reaction. Perform a flame test on the collected gas. Record the results. Keep heating until no more gas is produced. Mass of cooled test tube after heating = ______ g

Part 2 – HCl(aq) + Zn Set up another inverted test tube of water in the gas collecting tray. Place a small piece of mossy zinc in a test tube. Add 10 m. L of HCl and stopper the reaction test tube and begin to collect the gas. Perform a flame test on the gas. Record the results.

Analysis – 1. Write a skeleton equation for part 1. Water, sodium carbonate and a gas (that you tested) were produced. 2. Write a skeleton equation for part 2. Zinc chloride and a ‘popping’ gas was produced. 3. Balance the equations in analysis 1 and 2 4. Classify equation 1 and 2 into one of the five reaction types we have discussed. 5. Calculate the mass difference in the test tube for part 1. 6. Why is the final mass of the test tube in part 1 different from the beginning? Results –
- Slides: 5