Lab 11 EDTA Titration of Ca 2 and

Lab 11: EDTA Titration of Ca 2+ and Mg 2+ in Natural Waters By: Samantha Cabrera

Background Natural water contains some dissolved minerals; the two most common ones are calcium and magnesium ions. These ions can interfere with the ability of soaps and detergents to clean, reduce the quality of water that we drink, create soap scum, etc. (from http: //www. sjsu. edu/faculty/chem 55/55 mgca. htm)

Purpose To determine the concentration of calcium and magnesium ions (also known as “hardness”) by complexometric titration with EDTA.
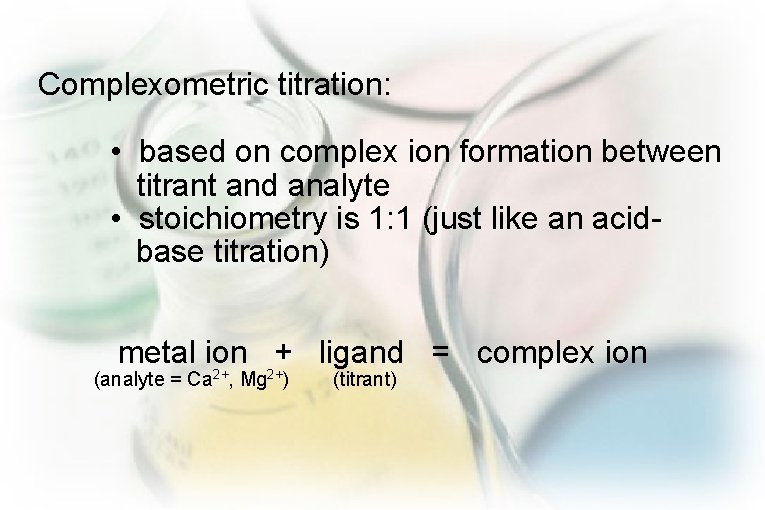
Complexometric titration: • based on complex ion formation between titrant and analyte • stoichiometry is 1: 1 (just like an acidbase titration) metal ion + ligand = complex ion (analyte = Ca 2+, Mg 2+) (titrant)
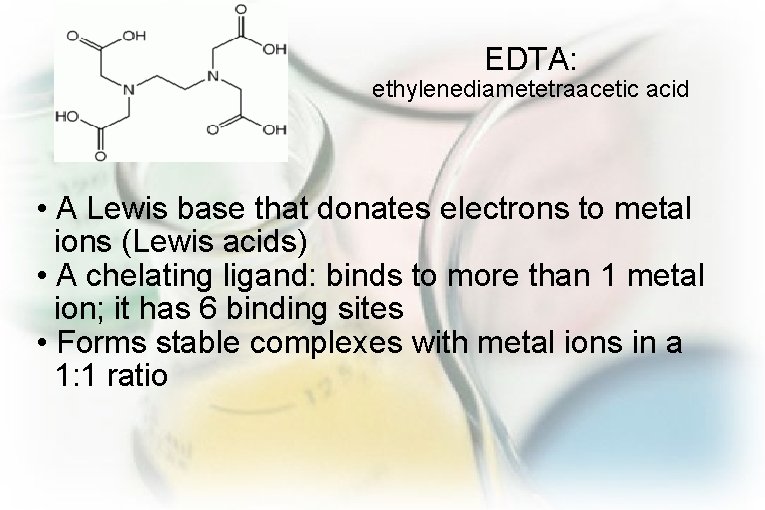
EDTA: ethylenediametetraacetic acid • A Lewis base that donates electrons to metal ions (Lewis acids) • A chelating ligand: binds to more than 1 metal ion; it has 6 binding sites • Forms stable complexes with metal ions in a 1: 1 ratio
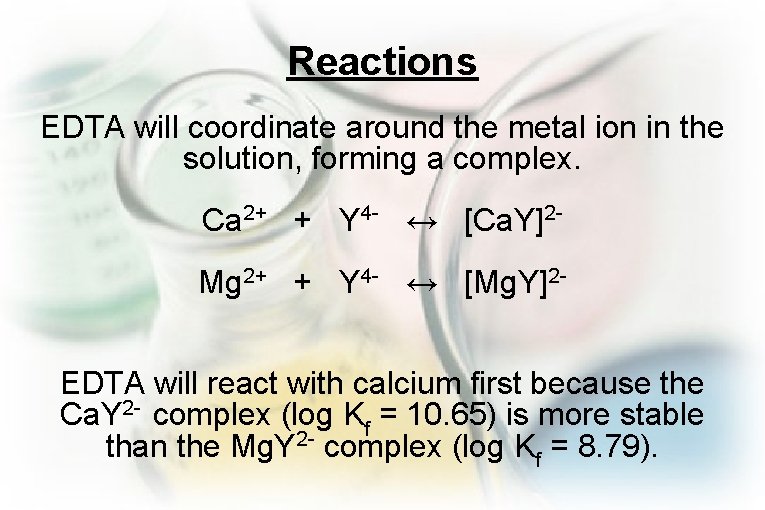
Reactions EDTA will coordinate around the metal ion in the solution, forming a complex. Ca 2+ + Y 4 - ↔ [Ca. Y]2 Mg 2+ + Y 4 - ↔ [Mg. Y]2 EDTA will react with calcium first because the Ca. Y 2 - complex (log Kf = 10. 65) is more stable than the Mg. Y 2 - complex (log Kf = 8. 79).
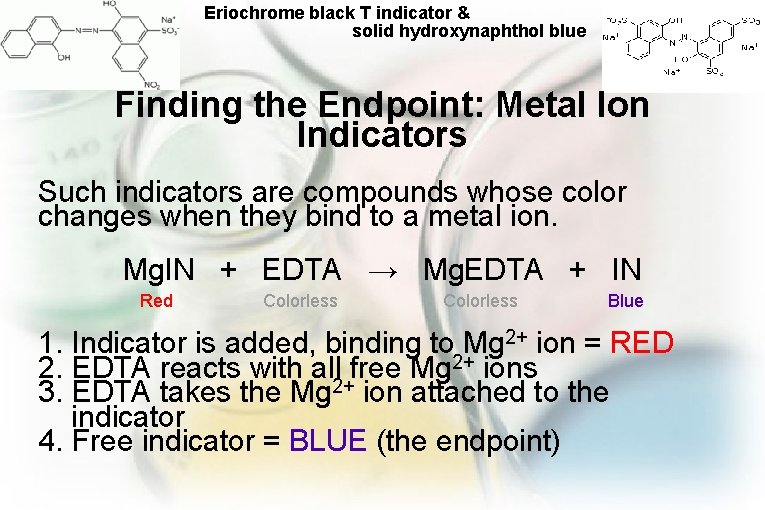
Eriochrome black T indicator & solid hydroxynaphthol blue Finding the Endpoint: Metal Ion Indicators Such indicators are compounds whose color changes when they bind to a metal ion. Mg. IN + EDTA → Mg. EDTA + IN Red Colorless Blue 1. Indicator is added, binding to Mg 2+ ion = RED 2. EDTA reacts with all free Mg 2+ ions 3. EDTA takes the Mg 2+ ion attached to the indicator 4. Free indicator = BLUE (the endpoint)
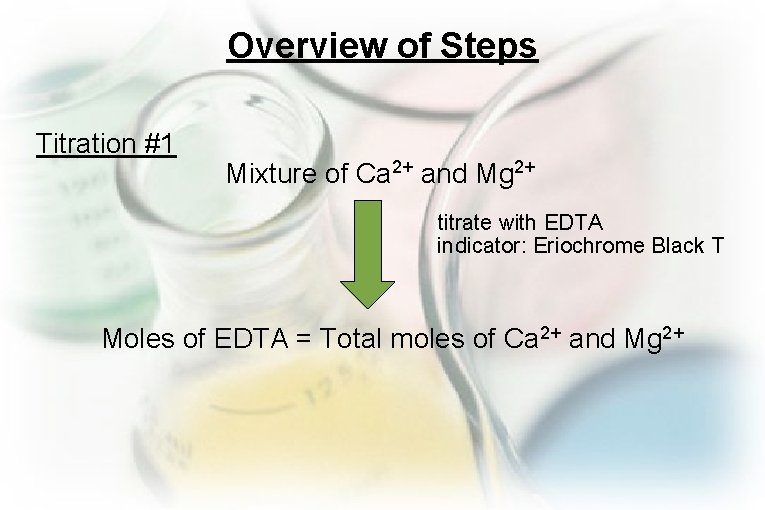
Overview of Steps Titration #1 Mixture of Ca 2+ and Mg 2+ titrate with EDTA indicator: Eriochrome Black T Moles of EDTA = Total moles of Ca 2+ and Mg 2+
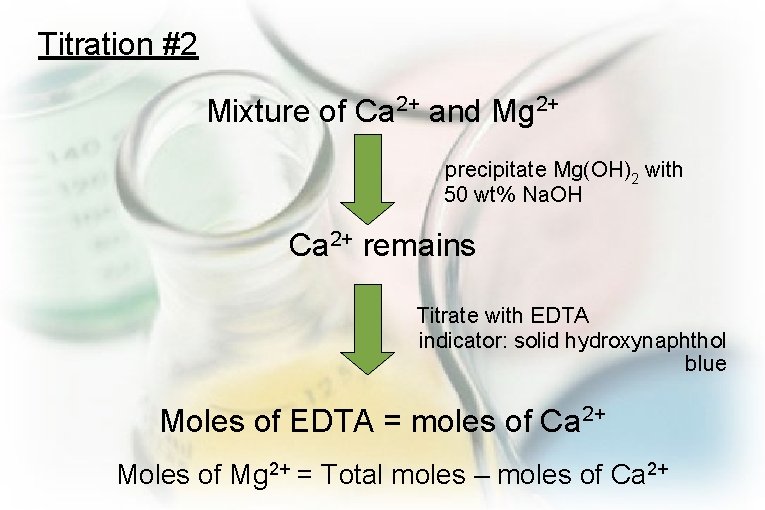
Titration #2 Mixture of Ca 2+ and Mg 2+ precipitate Mg(OH)2 with 50 wt% Na. OH Ca 2+ remains Titrate with EDTA indicator: solid hydroxynaphthol blue Moles of EDTA = moles of Ca 2+ Moles of Mg 2+ = Total moles – moles of Ca 2+
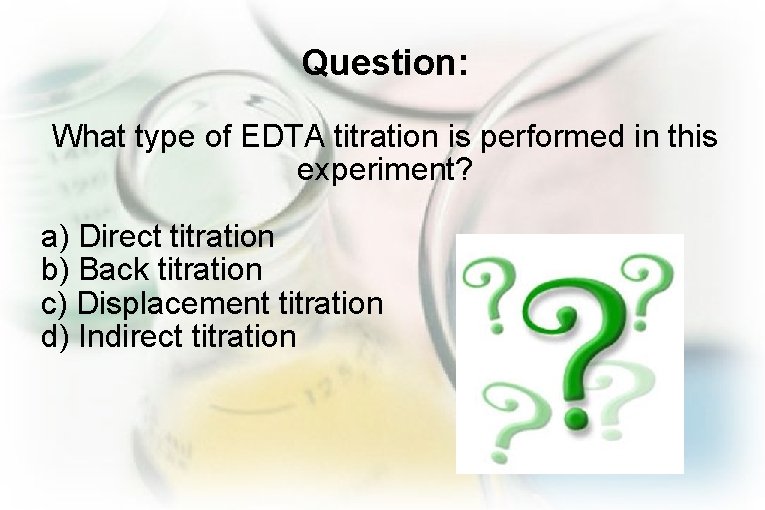
Question: What type of EDTA titration is performed in this experiment? a) Direct titration b) Back titration c) Displacement titration d) Indirect titration

Answer: a) Direct titration The analyte (Ca 2+ and Mg 2+) is directly titrated with the EDTA.
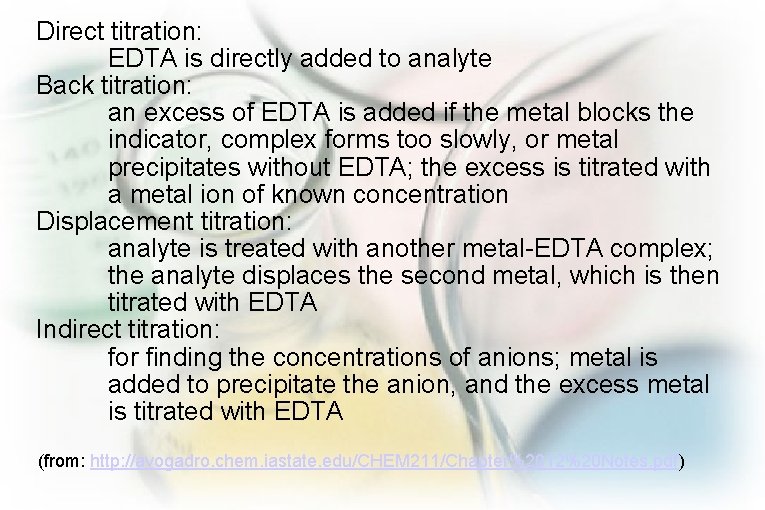
Direct titration: EDTA is directly added to analyte Back titration: an excess of EDTA is added if the metal blocks the indicator, complex forms too slowly, or metal precipitates without EDTA; the excess is titrated with a metal ion of known concentration Displacement titration: analyte is treated with another metal-EDTA complex; the analyte displaces the second metal, which is then titrated with EDTA Indirect titration: for finding the concentrations of anions; metal is added to precipitate the anion, and the excess metal is titrated with EDTA (from: http: //avogadro. chem. iastate. edu/CHEM 211/Chapter%2012%20 Notes. pdf)
- Slides: 12