LAB 10 Microbial enzymatic activity Amylase production test

LAB 10 Microbial enzymatic activity
Amylase production test - Starch agar is a differential medium that tests the ability of an organism to produce certain exoenzymes, including α-amylase and oligo-1, 6 -glucosidase, that hydrolyze starch. - Starch molecules are too large to enter the bacterial cell, so some bacteria secrete exoenzymes to degrade starch into subunits that can then be utilized by the organism. - Starch agar is a simple nutritive medium with starch added. Since no color change occurs in the medium when organisms hydrolyze starch, we add iodine to the plate after incubation. Iodine turns blue, purple, or black (depending on the concentration of iodine) in the presence of starch. - A clearing around the bacterial growth indicates that the organism has hydrolyzed starch.

Procedures - Streak the test organism across a small portion of the agar surface. - Incubate at 37 o. C for 48 hours. - Cover the surface with iodine. Rotate to distribute the iodine into a thin layer. Do not flood the plate. - Iodine will turn blue when it reacts with starch. A clear zone will be seen where starch has been digested.
Starch agar before inoculation
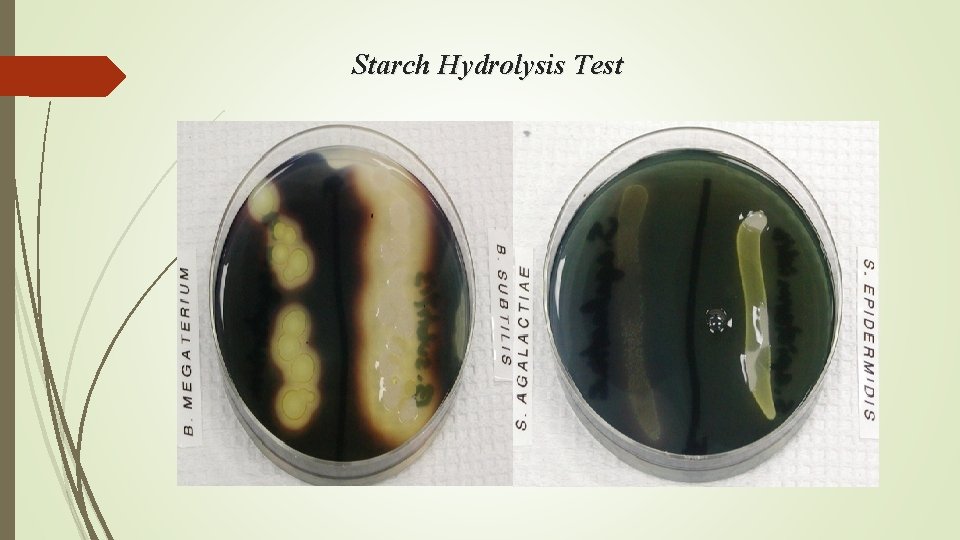
Starch Hydrolysis Test

Bacillus subtilis colony on a culture medium containing starch. The culture plate has been flooded with a weak iodine solution, which reveals a zone of clearing around the colony (arrow). This zone represents the area where the starch has been hydrolyzed so that it is no longer available to react with the iodine solution.
Catalase Activity Catalase production and activity can be detected by adding the substrate H 2 O 2 to an appropriately incubated(18 - to 24 -hour) tryptic soy agar slant culture. If catalase was produced by the bacteria, the above chemical reaction will liberate free O 2 gas. Bubbles of O 2 represent a positive catalase test; the absence of bubble formation is a negative catalase test. Catalase activity is very useful in differentiating between groups of bacteria. For example, the morphologically similar Enterococcus (catalase negative) and Staphylococcus (catalase positive) can be differentiated using the catalase test.

Procedure 1. Label each of the tryptic soy agar slants with the name of the bacterium to be inoculated, your name, and date. 2. Using aseptic technique, heavily inoculate each experimental bacterium into its appropriately labeled tube by means of a streak inoculation. 3. Incubate the slants at 35°C for 18 to 24 hours. 4. Remove growth from a slant using a wooden applicator stick or Nichrome wire loop and place the growth on a glass slide. The cells are then mixed in a drop of 3% H 2 O 2 or a drop of Difco’s Spot Test catalase reagent. Immediate bubbling indicates a positive catalase test.

Procedure Place a small amount of a bacterial colony (18 to 24 hours old) on a clean glass slide. Add one to two drops of 3% hydrogen peroxide. � Positive: � Negative: Rapid bubble formation No bubble formation
- Slides: 9