L 25 Electricity Magnetism 2 static electricity the
![L 25 Electricity & Magnetism [2] • static electricity – the charging process – L 25 Electricity & Magnetism [2] • static electricity – the charging process –](https://slidetodoc.com/presentation_image_h/94e386db5eedaa5e76b0ecf2cd921944/image-1.jpg)















- Slides: 25
![L 25 Electricity Magnetism 2 static electricity the charging process L 25 Electricity & Magnetism [2] • static electricity – the charging process –](https://slidetodoc.com/presentation_image_h/94e386db5eedaa5e76b0ecf2cd921944/image-1.jpg)
L 25 Electricity & Magnetism [2] • static electricity – the charging process – the van de Graff generator – electrostatic shielding • lightning • batteries and frogs legs • electric circuits 1

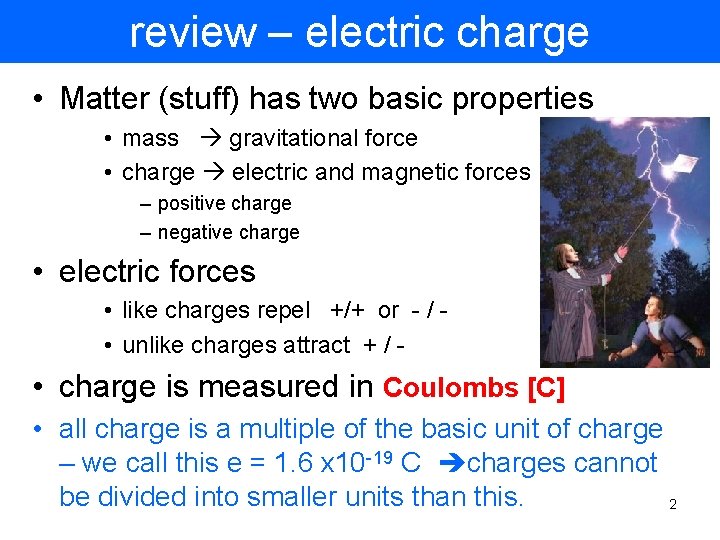
review – electric charge • Matter (stuff) has two basic properties • mass gravitational force • charge electric and magnetic forces – positive charge – negative charge • electric forces • like charges repel +/+ or - / • unlike charges attract + / - • charge is measured in Coulombs [C] • all charge is a multiple of the basic unit of charge – we call this e = 1. 6 x 10 -19 C charges cannot be divided into smaller units than this. 2

One Coulomb is a HUGE charge • To get a charge of one Coulomb on an object we would have to remove 6. 250 x 1018 electrons from it! • In the capacitor discharge demo, only 0. 01 C of charge were involved. 3

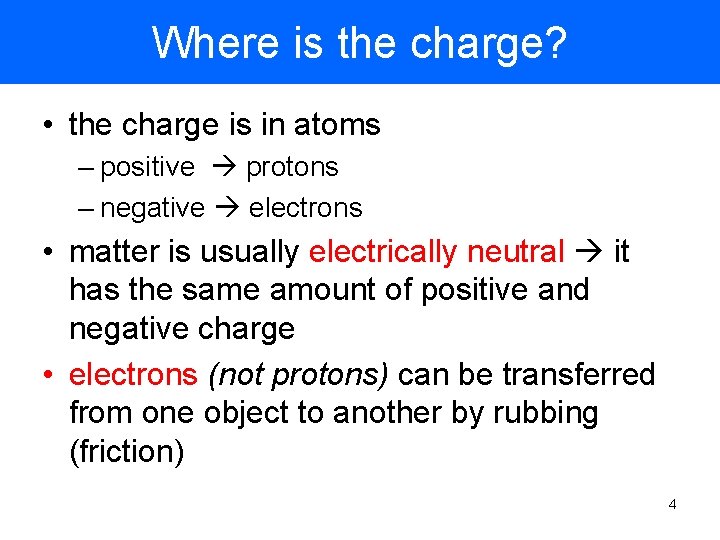
Where is the charge? • the charge is in atoms – positive protons – negative electrons • matter is usually electrically neutral it has the same amount of positive and negative charge • electrons (not protons) can be transferred from one object to another by rubbing (friction) 4

Charging by friction (triboelectric effect) • If you rub plastic with cat’s fur, electrons are rubbed onto the plastic making it negative • if you rub glass or plastic with silk, electrons are rubbed off the glass making it positive • the charge can be transferred to other objects. • only the electrons can be transferred 5

The charging process • an object is charged positive (has a net positive charge ) if electrons are removed from it • an object is charged negative (has a net negative charge) if electrons are transferred to it • charges can be transferred from conductors or non-conductors, but they can only move through conductors. 6

Example • Object A has a charge of -5 C and object B has a charge of +5 C. If -10 Coulombs of negative charge are transferred from object A to object B. What is the final charge on each object? -5 C -10 C A +5 C B • ANSWER: Object A has a net charge of +5 C and object B has a net charge of -5 C. +5 C – 5 C A B • Note that the net charge (=0) is the same before and after 7

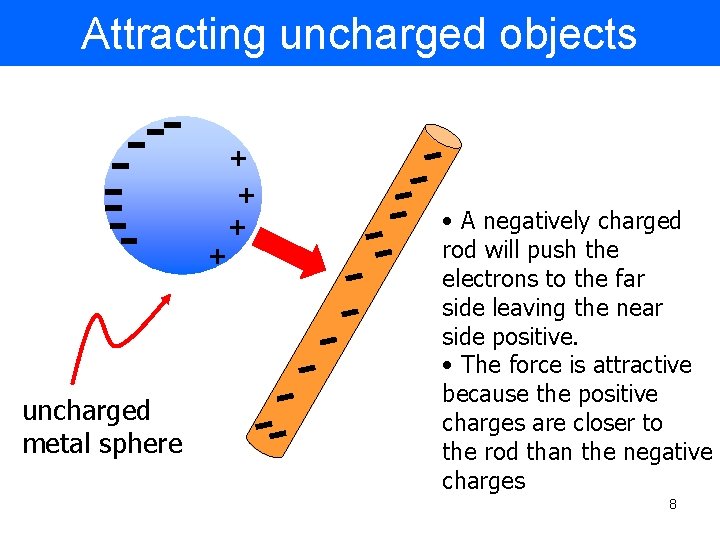
Attracting uncharged objects + + uncharged metal sphere • A negatively charged rod will push the electrons to the far side leaving the near side positive. • The force is attractive because the positive charges are closer to the rod than the negative charges 8

Can attract nonconductors also • Even though nonconductors do not have free electrons that can move around, the molecules can be polarized – the positive and negative charges can be separated slightly ++ ++ 9

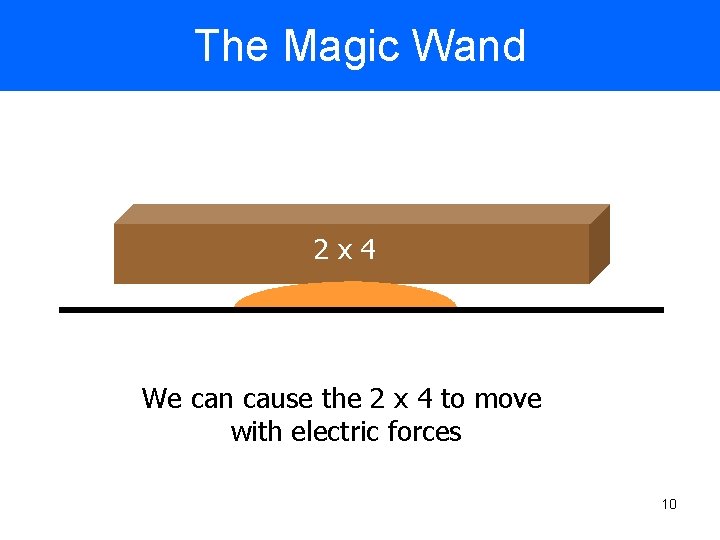
The Magic Wand 2 x 4 We can cause the 2 x 4 to move with electric forces 10

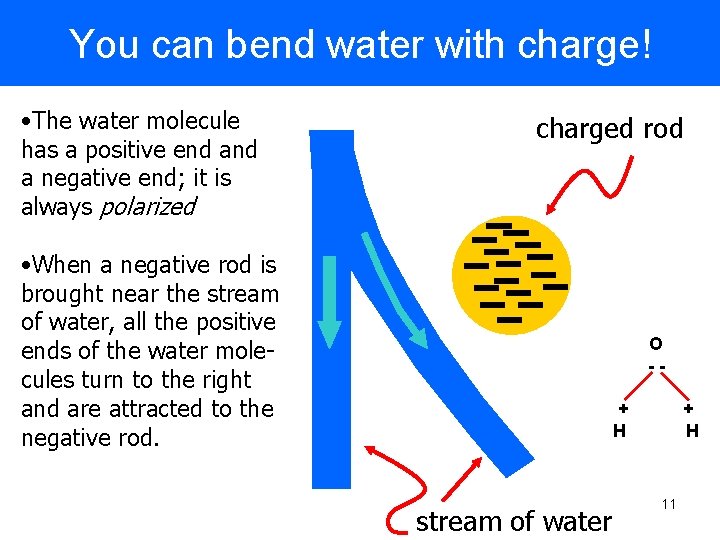
You can bend water with charge! • The water molecule has a positive end a negative end; it is always polarized charged rod • When a negative rod is brought near the stream of water, all the positive ends of the water molecules turn to the right and are attracted to the negative rod. O -+ H stream of water + H 11

Seeing the effects of charge: the electroscope • the electroscope is a simple device for observing the presence of electric charge • it consists of a small piece of metal foil (gold if possible) suspended from a rod with a metal ball at its top • If a negatively charged rod is placed near the ball, the electrons move away because of the repulsion. 12 The two sides of the metal foil then separate.

Danger High Voltage ! • The van de Graff can charge the sphere to more than 50, 000 volts! • This is enough to cause discharges to the surrounding air ionization or breakdown • The sparks excite air molecules which give off light 13

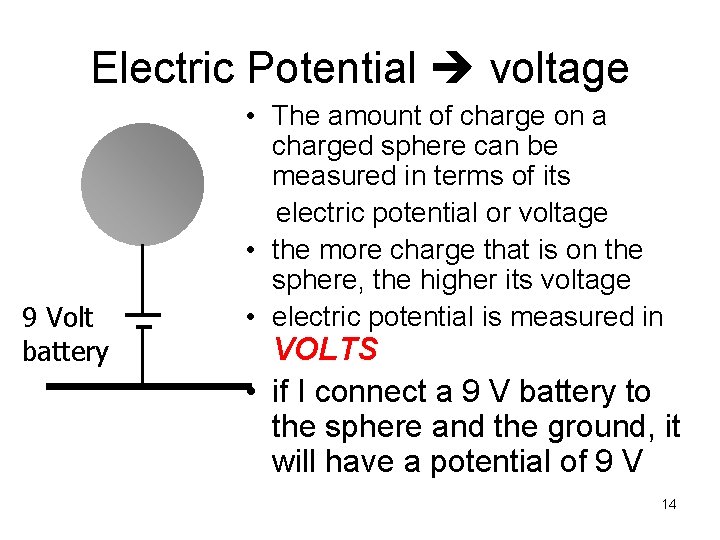
Electric Potential voltage 9 Volt battery • The amount of charge on a charged sphere can be measured in terms of its electric potential or voltage • the more charge that is on the sphere, the higher its voltage • electric potential is measured in VOLTS • if I connect a 9 V battery to the sphere and the ground, it will have a potential of 9 V 14

Lightning- outdoor spark n n n causes 80 million dollars in damage each year in the US On average, kills 90 people a year in the US lasts only a thousandth of a second carries up to 200, 000 A causes the thunder! 15

development of a lightning bolt charge separation stepped leader & streamer leader meets streamer lightning bolt 16

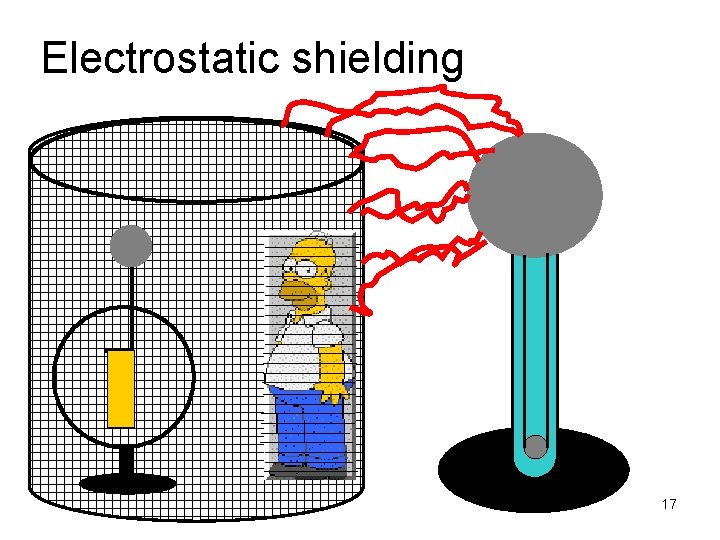
Electrostatic shielding 17

Electrostatic shielding • The effect of the high voltage on the van de Graff generator stops on the outside of the metal cage Homer is SAFE! • Being inside your car during a lightning storm offers you some protection • radio signals cannot penetrate through a metal enclosure • the metal bars (rebar) that reinforce the concrete in walls can also interfere 18

Conductors and Non- Conductors Metals (copper, aluminum, iron) are conductors of electricity they allow current (moving free electrons) to pass through them Plastics, wood, ceramics, and glass are non-conductors (or insulators) they do not let electricity flow through them they have no free electrons to move around 19

Pure water is non-conducting • clean water will not conduct electricity • if salt or acid is added, however, it will conduct electricity H 2 O carbon electrodes 20

A salt water solution is a conductor • When salt Na. Cl (sodium chloride) is added to water H 2 O, the Na. Cl molecule dissociates into a positive ion Na+, and a negative ion Cl-. • Thus the solutions contains both positive and negative ions, both of which can conduct electricity. • Electric current can pass through dirty bath water and through you also! • we are conductors – water + Na+ + Cl– 21

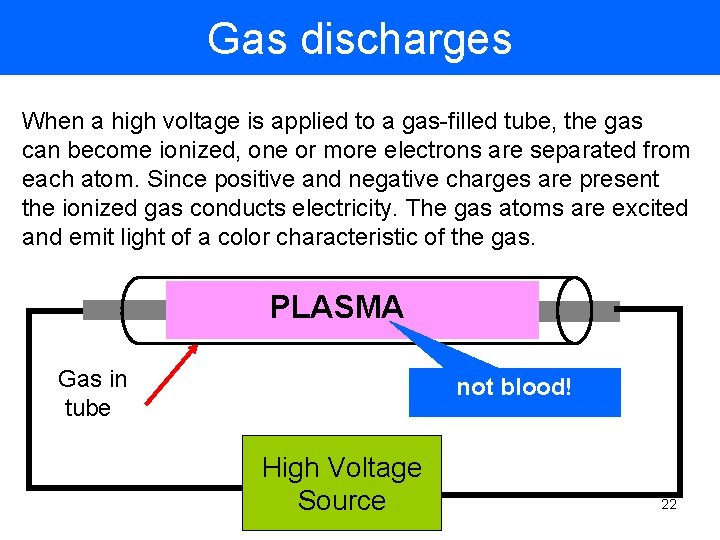
Gas discharges When a high voltage is applied to a gas-filled tube, the gas can become ionized, one or more electrons are separated from each atom. Since positive and negative charges are present the ionized gas conducts electricity. The gas atoms are excited and emit light of a color characteristic of the gas. PLASMA Gas in tube not blood! High Voltage Source 22

examples of electrical discharges the Aurora fluorescent lamp neon lights 23

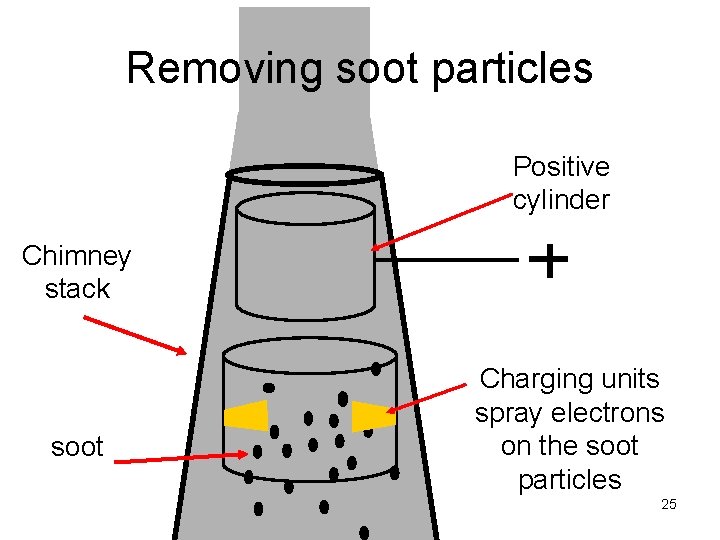
applications of electrostatics • Xerox copiers use electrostatic attraction to put the ink droplets on the paper • electrostatic precipitators use the attraction of charged dust to remove dust particles from smoke. • can be used to hold balloons on your head 24

Removing soot particles Positive cylinder Chimney stack soot Charging units spray electrons on the soot particles 25
 Static electricity and current electricity
Static electricity and current electricity Current electricity gif
Current electricity gif Electricity and magnetism vocabulary
Electricity and magnetism vocabulary Electricity and magnetism jeopardy
Electricity and magnetism jeopardy Grade 5 electricity and magnetism
Grade 5 electricity and magnetism 4 forces of nature
4 forces of nature Electricity and magnetism
Electricity and magnetism Electricity and magnetism
Electricity and magnetism Sph3u electricity and magnetism
Sph3u electricity and magnetism Electricity and magnetism
Electricity and magnetism Ib physics topic 5 question bank
Ib physics topic 5 question bank Electromagnet experiment hypothesis
Electromagnet experiment hypothesis Electricity and magnetism
Electricity and magnetism Magnetism
Magnetism Magnetism and electricity
Magnetism and electricity Ampere
Ampere Static electricity.
Static electricity. Bill nye friction worksheet
Bill nye friction worksheet What is static electricity
What is static electricity Graphic organizer 1 electric current
Graphic organizer 1 electric current Static electricity
Static electricity Chapter 20 static electricity answers
Chapter 20 static electricity answers Is cotton negatively charged
Is cotton negatively charged Static electricity examples
Static electricity examples Static electricity quotes
Static electricity quotes U in electricity
U in electricity