L 18 Thermodynamics 3 Heat transfer convection conduction
![L 18 Thermodynamics [3] • Heat transfer • convection • conduction • radiation • L 18 Thermodynamics [3] • Heat transfer • convection • conduction • radiation •](https://slidetodoc.com/presentation_image/f910861aede9950df6a4e71464f443b4/image-1.jpg)














- Slides: 32
![L 18 Thermodynamics 3 Heat transfer convection conduction radiation L 18 Thermodynamics [3] • Heat transfer • convection • conduction • radiation •](https://slidetodoc.com/presentation_image/f910861aede9950df6a4e71464f443b4/image-1.jpg)
L 18 Thermodynamics [3] • Heat transfer • convection • conduction • radiation • • emitters of radiation seeing behind closed doors Greenhouse effect global warming

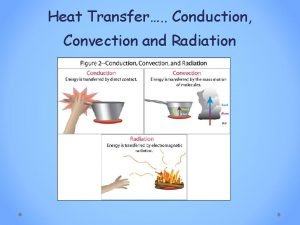
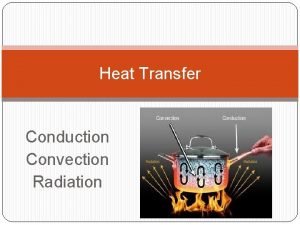
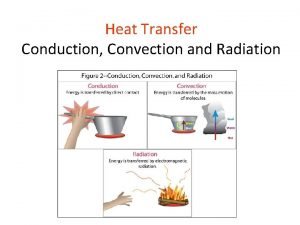
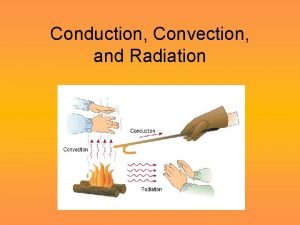
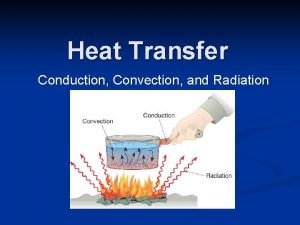
Heat flow • HEAT the energy that flows from one system to another because of temperature differences. • But how does it flow? Three ways: • convection • conduction • radiation

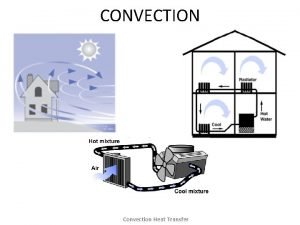
Convection • heat is carried from place to place by the bulk movement of either liquids or gases • does not apply to solids • when water is boiled, hot liquid rises and mixes with cooler liquid, thus the heat is transferred • Hot air rises: • want heat into lower level of house (winter) • cooled air into upper levels (summer)

Conduction • heat is transferred directly through a material, with no bulk movement of stuff • only energy moves iron is a particularly poor conductor of heat

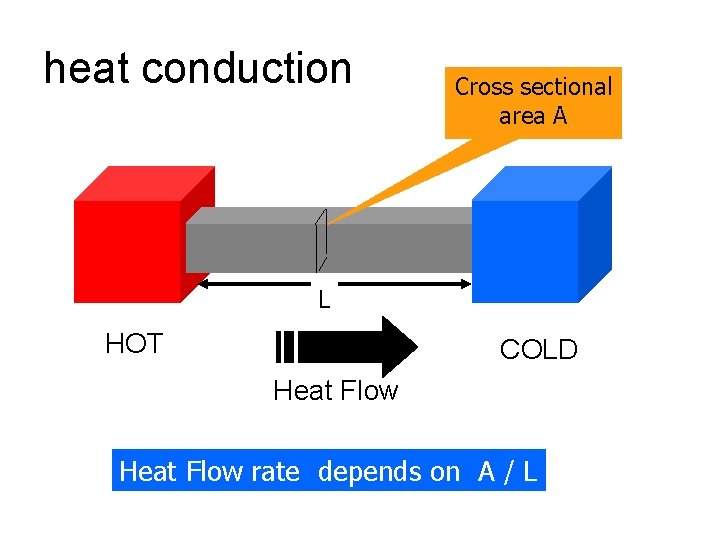
heat conduction Cross sectional area A L HOT COLD Heat Flow rate depends on A / L

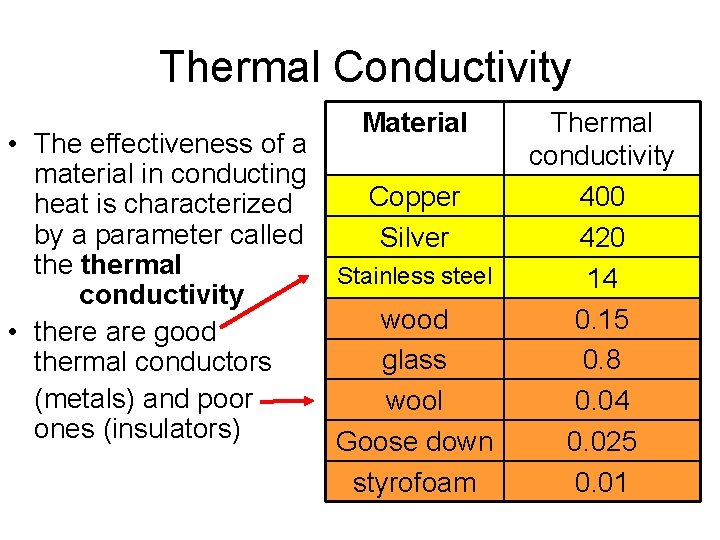
Thermal Conductivity Material • The effectiveness of a material in conducting Copper heat is characterized by a parameter called Silver thermal Stainless steel conductivity wood • there are good glass thermal conductors (metals) and poor wool ones (insulators) Goose down styrofoam Thermal conductivity 400 420 14 0. 15 0. 8 0. 04 0. 025 0. 01

Thermal Conductivities of Metals Metal Silver Thermal Conductivity (W/m K) 406 Copper 385 Aluminum 205 Brass 109 Iron 80 Steel 50

Grandma’s silver spoons

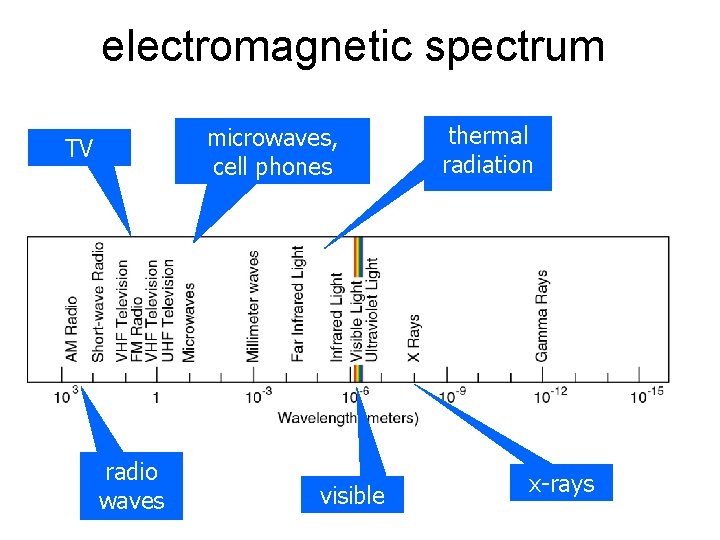
radiation • Radiation is the heat transfer by electromagnetic waves – thermal light waves - invisible to the eyes • thermal radiation is a small part of the electromagnetic spectrum – waves are characterized by their frequency or wavelength • different colors in the visible correspond to different wavelengths from red to blue

electromagnetic spectrum microwaves, cell phones TV radio waves visible thermal radiation x-rays

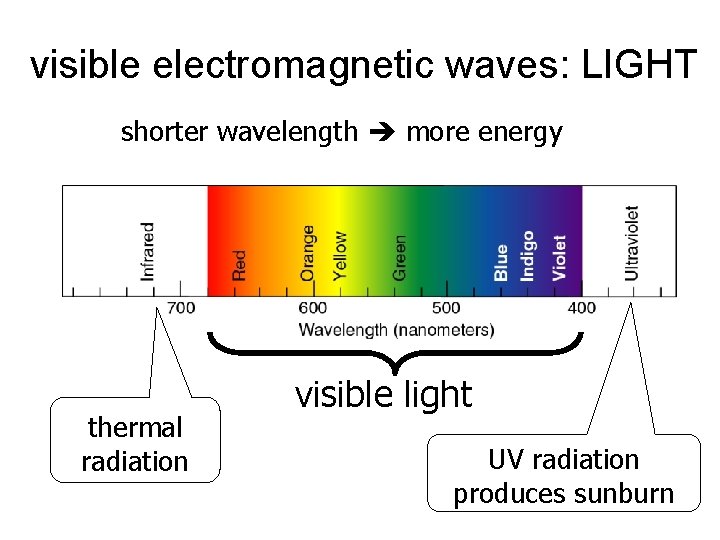
visible electromagnetic waves: LIGHT shorter wavelength more energy thermal radiation visible light UV radiation produces sunburn

Thermal Radiation • The warmth you feel from the sun is the sun’s thermal radiation • It travels through the vacuum of space to reach earth, no material is necessary (takes 8 minutes) • you can feel its effects even though you cannot see the radiation. • you can feel thermal radiation from a fireplace

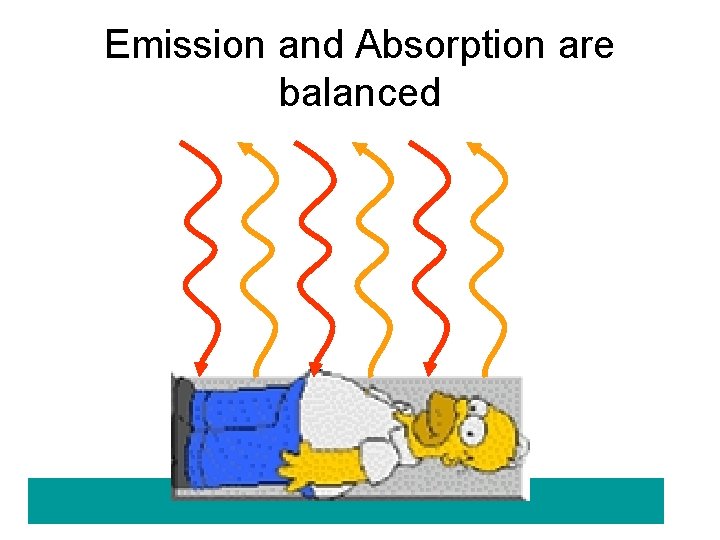
What produces thermal radiation? • all objects whose temperature is above absolute zero emit thermal radiation • The hotter the object, the more radiation it emits, the amount of radiation is ~ T 4 • We all continuously emit thermal radiation • We also absorb it from objects and people around us • If we just emitted radiation we would eventually cool to absolute zero!

Emission and Absorption are balanced

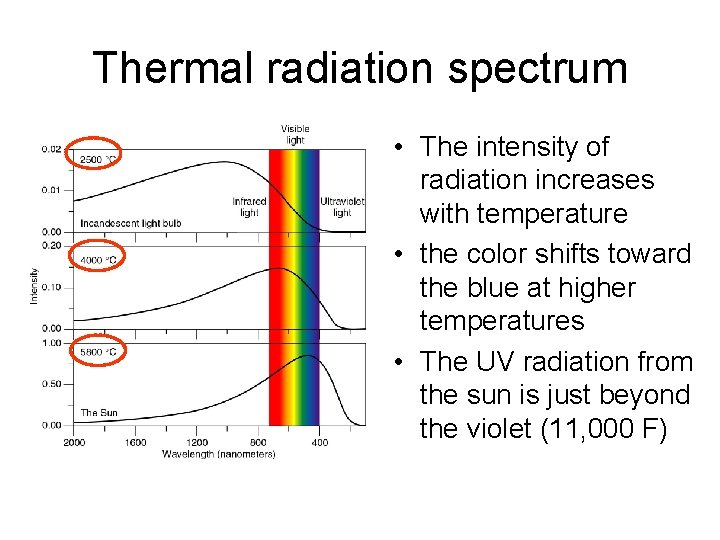
Thermal radiation spectrum • The intensity of radiation increases with temperature • the color shifts toward the blue at higher temperatures • The UV radiation from the sun is just beyond the violet (11, 000 F)

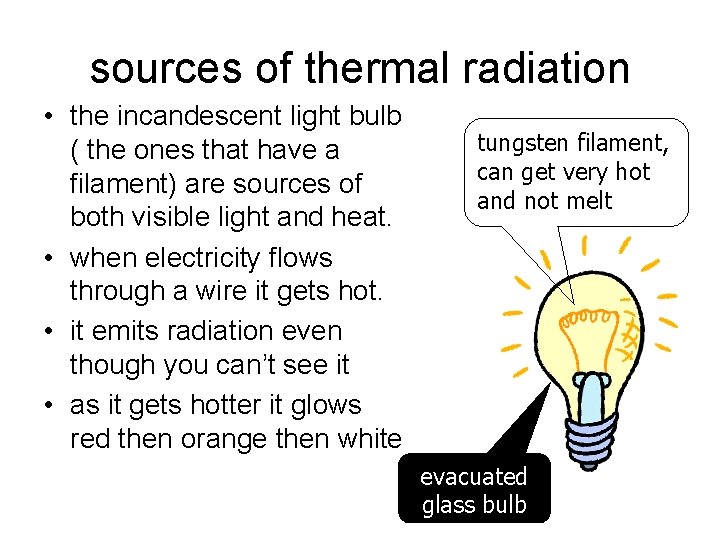
sources of thermal radiation • the incandescent light bulb ( the ones that have a filament) are sources of both visible light and heat. • when electricity flows through a wire it gets hot. • it emits radiation even though you can’t see it • as it gets hotter it glows red then orange then white tungsten filament, can get very hot and not melt evacuated glass bulb

Radiation emitted by hot objects • The hotter they are, the more they emit • the efficiency with which an object emits thermal radiation is characterized be a parameter called its emissivity e • e is a number between 0 and 1 • a good emitter has an e close to 1 • a poor emitter has an e close to 0

good emitters are good absorbers • an object that is a good emitter is also a good absorber of thermal radiation • a poor emitter is also a poor absorber • generally dark, dull objects are the best emitters/absorbers • shinny objects are poor emitters/absorbers, they are good reflectors of radiation • If you do not want the edges of your pie to burn, you wrap it in aluminum foil

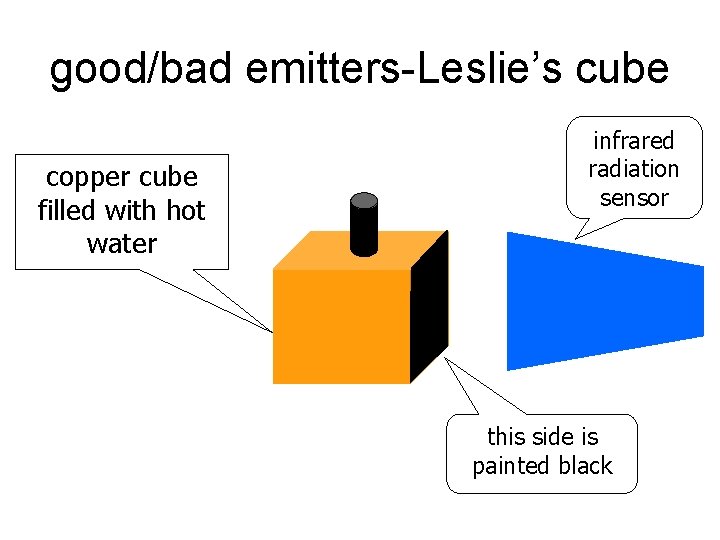
good/bad emitters-Leslie’s cube copper cube filled with hot water infrared radiation sensor this side is painted black

Practical considerations • wear light clothing in summer light clothing absorbs less sunlight • cover all body parts in winter warm body parts (like your head) emit radiation

thermal radiation • all objects that are at a temperature above absolute zero emit thermal radiation (waves) • the higher the temp, the more they emit • the color (wavelength) of the emitted waves goes from red orange yellow blue as the temperature increases

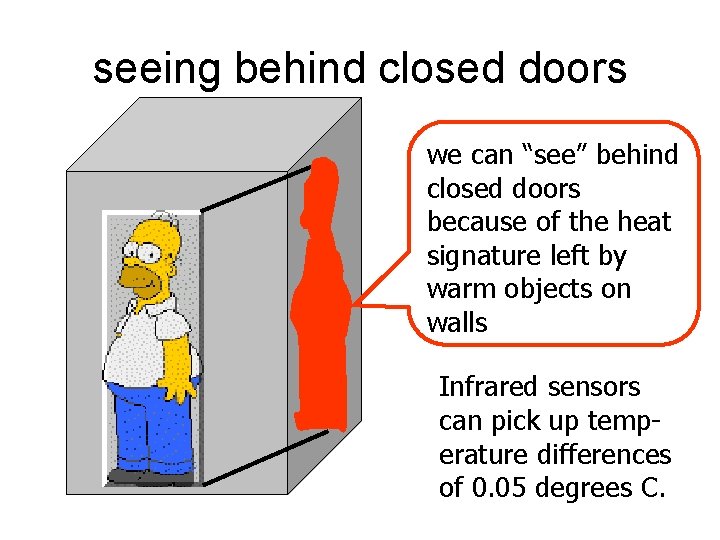
seeing behind closed doors we can “see” behind closed doors because of the heat signature left by warm objects on walls Infrared sensors can pick up temperature differences of 0. 05 degrees C.

Which one is best? A. silvered B. silvered and un-evacuated C. evacuated D. un-silvered and un-evacuated

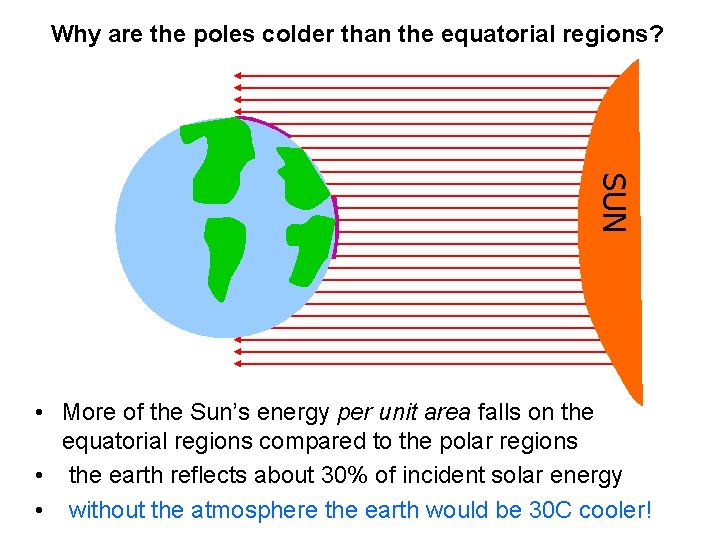
Why are the poles colder than the equatorial regions? SUN • More of the Sun’s energy per unit area falls on the equatorial regions compared to the polar regions • the earth reflects about 30% of incident solar energy • without the atmosphere the earth would be 30 C cooler!

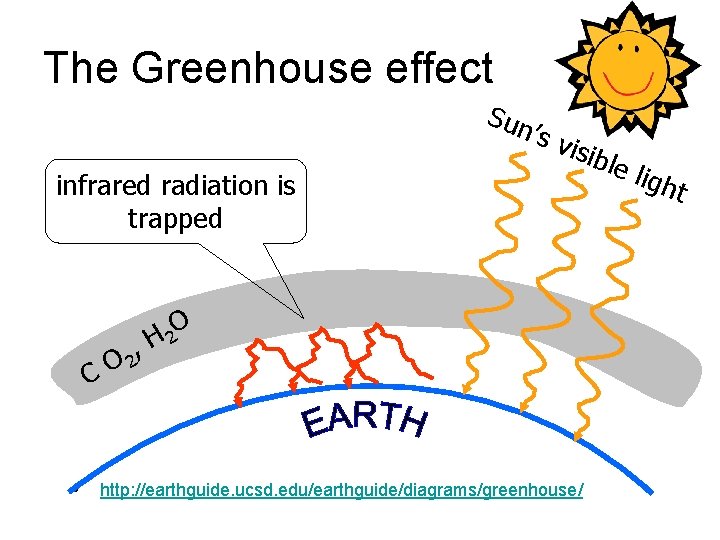
The Greenhouse effect Sun ’s infrared radiation is trapped C • , O 2 visi O H 2 http: //earthguide. ucsd. edu/earthguide/diagrams/greenhouse/ ble ligh t

Effect of greenhouse gases: H 2 O, CO 2, CH 4, . . . • the sun’s visible light can penetrate through the atmosphere to the earth’s surface where it heats it • the visible light energy is converted to thermal light energy • thermal radiation is reflected from the greenhouse gases in the atmosphere

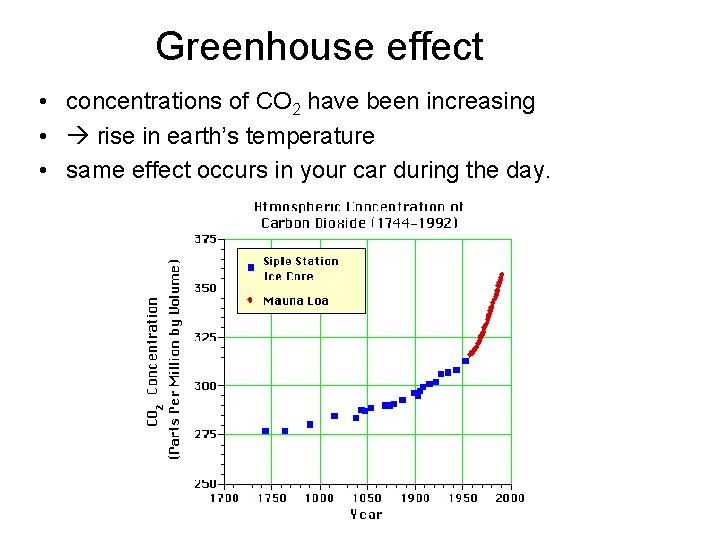
Greenhouse effect • concentrations of CO 2 have been increasing • rise in earth’s temperature • same effect occurs in your car during the day.

James Hansen • NASA Goddard Institute for Space Studies, Columbia University, NY • • Born 1941 Denison, IA Ph. D 1967 Univ. Iowa MS 1965 Univ. Iowa BA 1963 Univ. Iowa http: //www. columbia. edu/~jeh 1/dots_feb 2007. ppt

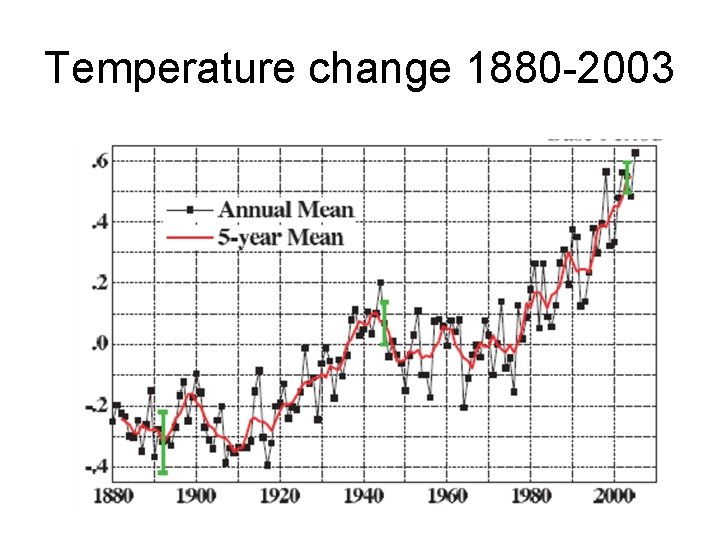
Temperature change 1880 -2003

1960 -2020

Global warming issues • 88, 800, 000 sites on Google • • http: //www. hillsdale. edu/news/imprimis/archive/issue. asp? year=2007&month=08 http: //www. epa. gov/climatechange/ • http: //ipcc-wg 1. ucar. edu/wg 1 -report. html • What is the effect of human activity (anthropogenic) on the buildup of greenhouse gases? • (NRC 2001) Because of the large and still uncertain level of natural variability inherent in the climate record and the uncertainties in the time histories of the various forcing agents (and particularly aerosols), a causal linkage between the buildup of greenhouse gases in the atmosphere and the observed climate changes during the 20 th century cannot be unequivocally established. • The IPCC (International Panel on Climate Change) 2/2/07: “global warming is “very likely” caused by man. • Drive less and convert to fluorescent lights

The ozone layer: a related, but different issue • ozone, O 3 is a naturally occurring trace element in the atmosphere • It absorbs solar ultraviolet radiation, especially the harmful UV-B rays • it is destroyed by Cfc’s (chlorofluorocarbons) • loss affects us and environment
 Radiation examples
Radiation examples Methods of heat transfer
Methods of heat transfer Transfer of heat conduction
Transfer of heat conduction What is heat transfer conduction convection and radiation
What is heat transfer conduction convection and radiation Convection heat transfer
Convection heat transfer Examples of convection
Examples of convection Convection heat transfer
Convection heat transfer Convection heat transfer equation
Convection heat transfer equation Heat transfer by conduction gizmo
Heat transfer by conduction gizmo Transient conduction means
Transient conduction means Example of radiation heat transfer
Example of radiation heat transfer Conduction formula
Conduction formula Heat transfer conduction
Heat transfer conduction Difference between conduction convection and radiation
Difference between conduction convection and radiation Temperature conduction convection radiation
Temperature conduction convection radiation Conduction convection radiation equations
Conduction convection radiation equations List two examples of convection
List two examples of convection Kinds of heat energy
Kinds of heat energy Temperature, conduction, convection, radiation. *
Temperature, conduction, convection, radiation. * Method of heat transfer
Method of heat transfer Conduction examples
Conduction examples Objectives of heat transfer
Objectives of heat transfer Conduction popcorn
Conduction popcorn Heat energy is transferred by conduction whenever molecules
Heat energy is transferred by conduction whenever molecules Conduction convection radiation examples
Conduction convection radiation examples Conduction vs convection
Conduction vs convection Venn diagram of conduction, convection and radiation
Venn diagram of conduction, convection and radiation Venn diagram of states of matter
Venn diagram of states of matter Whats conduction convection and radiation
Whats conduction convection and radiation Radiation convection and conduction
Radiation convection and conduction Bellringer conduction convection or radiation
Bellringer conduction convection or radiation Heat transfer by radiation
Heat transfer by radiation Trasnferin
Trasnferin