Ksp Solubility Product Chapter 16 Ksp background The

Ksp – Solubility Product Chapter 16
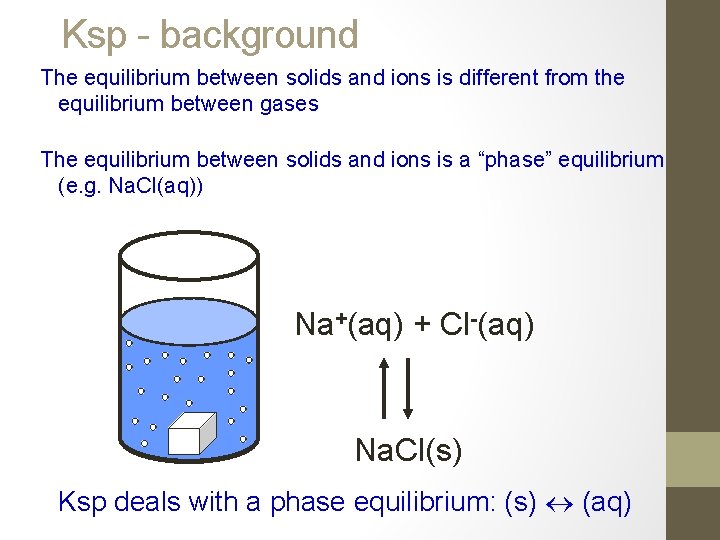
Ksp - background The equilibrium between solids and ions is different from the equilibrium between gases The equilibrium between solids and ions is a “phase” equilibrium (e. g. Na. Cl(aq)) Na+(aq) + Cl-(aq) Na. Cl(s) Ksp deals with a phase equilibrium: (s) (aq)
![Why use Ksp instead of Kc? Consider: Na. Cl Na+(aq) + Cl-(aq) [Na. Cl(s)] Why use Ksp instead of Kc? Consider: Na. Cl Na+(aq) + Cl-(aq) [Na. Cl(s)]](http://slidetodoc.com/presentation_image_h/074e584058b48ca66f46c8928428a99c/image-3.jpg)
Why use Ksp instead of Kc? Consider: Na. Cl Na+(aq) + Cl-(aq) [Na. Cl(s)] doesn’t [Na+(aq)] [Cl-(aq)] K= change (it’s a konstant) [Na. Cl(s)] K • [Na. Cl(s)] = [Na+(aq)] [Cl-(aq)] Ksp = [Na+(aq)] [Cl-(aq)] We use Ksp because it indicates that the + Na (aq) + Cl (aq) equilibrium is a phase equilibrium (between a solid and it’s ions) Na. Cl(s)

Ksp - molar solubility § A solid always dissolves until no more can dissolve - called molar solubility , s, (mol/L or M) § An equilibrium is established when the amount dissolving equals the amount precipitating § This can only be true if there is some solid (thus, we can usually see if there is an equilibrium) § A solution with solid remaining is called “saturated”…this means that the solution is at equilibrium

Relative solubilities • Ksp will only allow us to compare the solubility of solids that fall apart into the same number of ions. • The bigger the Ksp of those the more soluble.
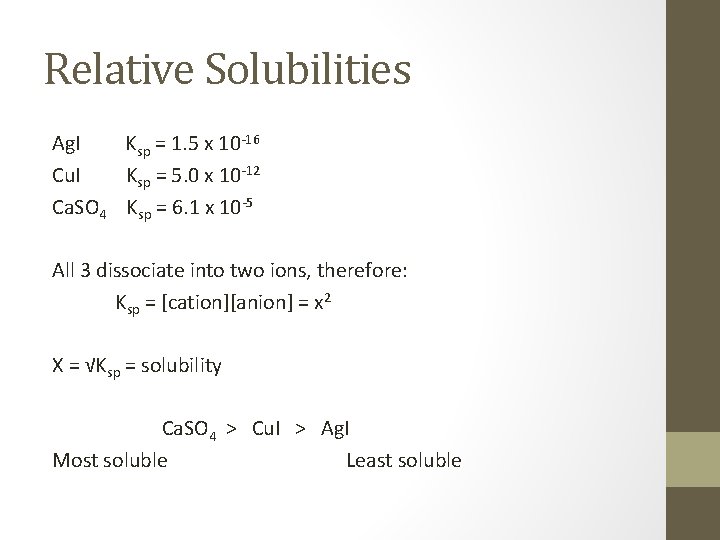
Relative Solubilities Ag. I Ksp = 1. 5 x 10 -16 Cu. I Ksp = 5. 0 x 10 -12 Ca. SO 4 Ksp = 6. 1 x 10 -5 All 3 dissociate into two ions, therefore: Ksp = [cation][anion] = x 2 X = √Ksp = solubility Ca. SO 4 > Cu. I > Ag. I Most soluble Least soluble
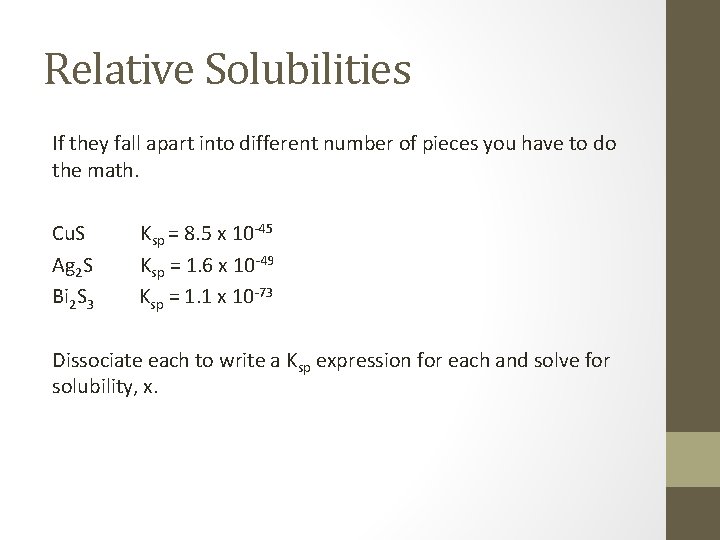
Relative Solubilities If they fall apart into different number of pieces you have to do the math. Cu. S Ag 2 S Bi 2 S 3 Ksp = 8. 5 x 10 -45 Ksp = 1. 6 x 10 -49 Ksp = 1. 1 x 10 -73 Dissociate each to write a Ksp expression for each and solve for solubility, x.
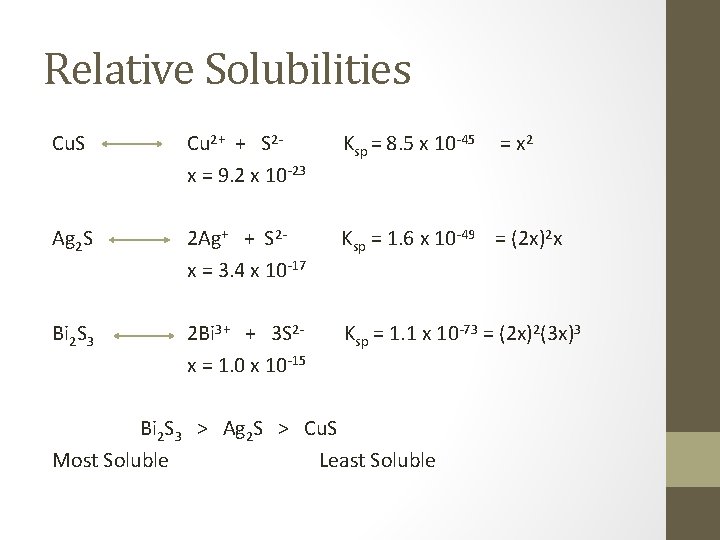
Relative Solubilities Cu. S Cu 2+ + S 2 x = 9. 2 x 10 -23 Ksp = 8. 5 x 10 -45 Ag 2 S 2 Ag+ + S 2 x = 3. 4 x 10 -17 Ksp = 1. 6 x 10 -49 = (2 x)2 x Bi 2 S 3 2 Bi 3+ + 3 S 2 x = 1. 0 x 10 -15 Ksp = 1. 1 x 10 -73 = (2 x)2(3 x)3 Bi 2 S 3 > Ag 2 S > Cu. S Most Soluble Least Soluble = x 2
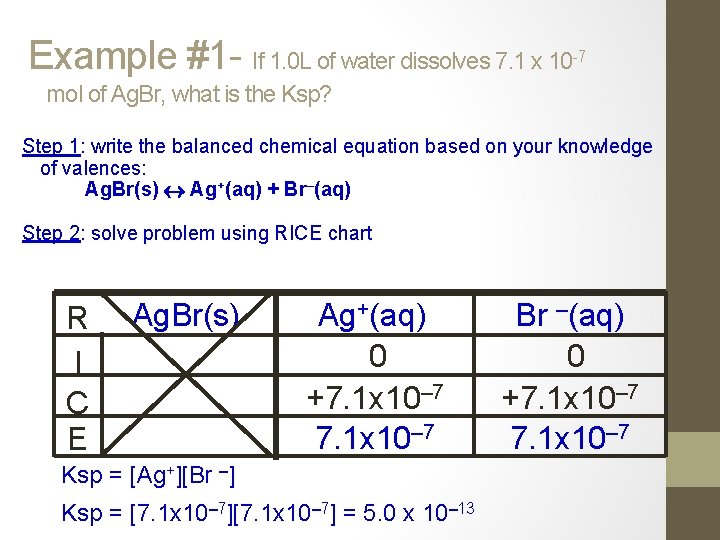
Example #1 - If 1. 0 L of water dissolves 7. 1 x 10 -7 mol of Ag. Br, what is the Ksp? Step 1: write the balanced chemical equation based on your knowledge of valences: Ag. Br(s) Ag+(aq) + Br–(aq) Step 2: solve problem using RICE chart R I C E Ag. Br(s) Ag+(aq) 0 +7. 1 x 10– 7 Ksp = [Ag+][Br –] Ksp = [7. 1 x 10– 7] = 5. 0 x 10– 13 Br –(aq) 0 +7. 1 x 10– 7
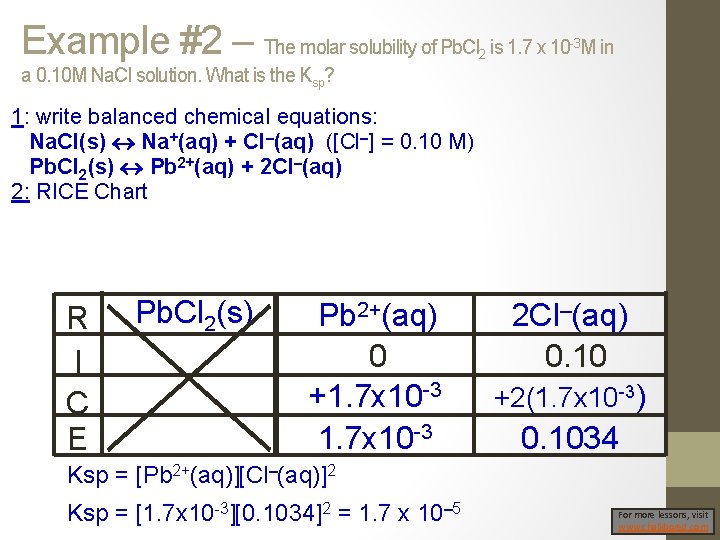
Example #2 – The molar solubility of Pb. Cl is 1. 7 x 10 M in 2 -3 a 0. 10 M Na. Cl solution. What is the Ksp? 1: write balanced chemical equations: Na. Cl(s) Na+(aq) + Cl–(aq) ([Cl–] = 0. 10 M) Pb. Cl 2(s) Pb 2+(aq) + 2 Cl–(aq) 2: RICE Chart R I C E Pb. Cl 2(s) Pb 2+(aq) 0 +1. 7 x 10 -3 2 Cl–(aq) 0. 10 +2(1. 7 x 10 -3) 0. 1034 Ksp = [Pb 2+(aq)][Cl–(aq)]2 Ksp = [1. 7 x 10 -3][0. 1034]2 = 1. 7 x 10– 5 For more lessons, visit www. chalkbored. com
- Slides: 10