Ksp background The equilibrium between solids and ions
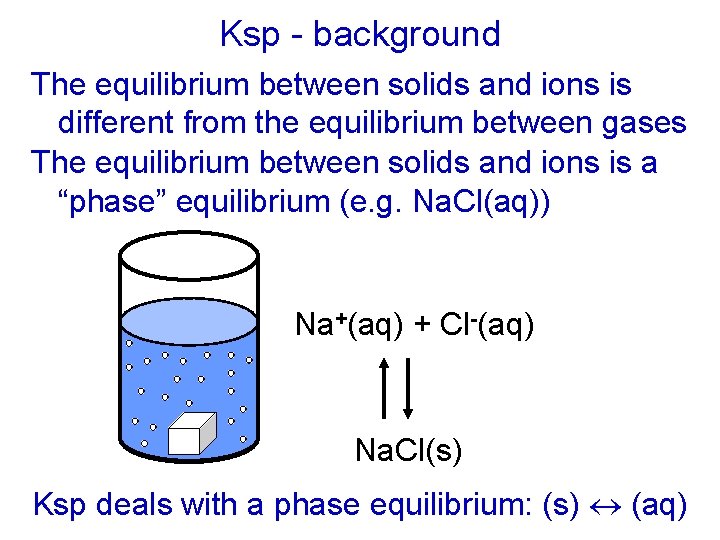
Ksp - background The equilibrium between solids and ions is different from the equilibrium between gases The equilibrium between solids and ions is a “phase” equilibrium (e. g. Na. Cl(aq)) Na+(aq) + Cl-(aq) Na. Cl(s) Ksp deals with a phase equilibrium: (s) (aq)
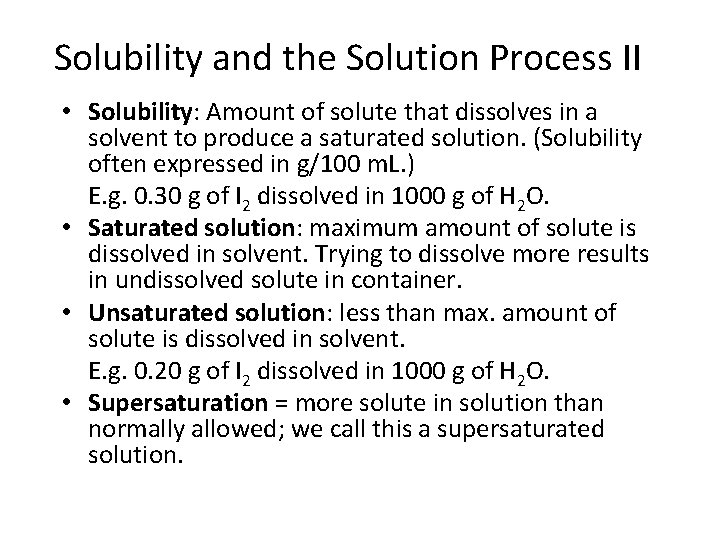
Solubility and the Solution Process II • Solubility: Amount of solute that dissolves in a solvent to produce a saturated solution. (Solubility often expressed in g/100 m. L. ) E. g. 0. 30 g of I 2 dissolved in 1000 g of H 2 O. • Saturated solution: maximum amount of solute is dissolved in solvent. Trying to dissolve more results in undissolved solute in container. • Unsaturated solution: less than max. amount of solute is dissolved in solvent. E. g. 0. 20 g of I 2 dissolved in 1000 g of H 2 O. • Supersaturation = more solute in solution than normally allowed; we call this a supersaturated solution.

Finding the solubility product Ksp First we need an equation To find the equation we need to know the dissolving equation Let’s use the example of Ca. Cl 2 The equation would be Ca. Cl 2(s) Ca+2(aq) + 2 Cl-(aq)

The equation would be Ca. Cl 2(s) Ca+2(aq) + 2 Cl-(aq) All equilibrium constants have the equation Constant = [Products] [Reactants] And unlike the rate equation equilibrium constants are raised to the power of the coefficient in the balanced equation The equilibrium balance is not effected by how much solid is left Ksp = [Ca+2]. [Cl-]2
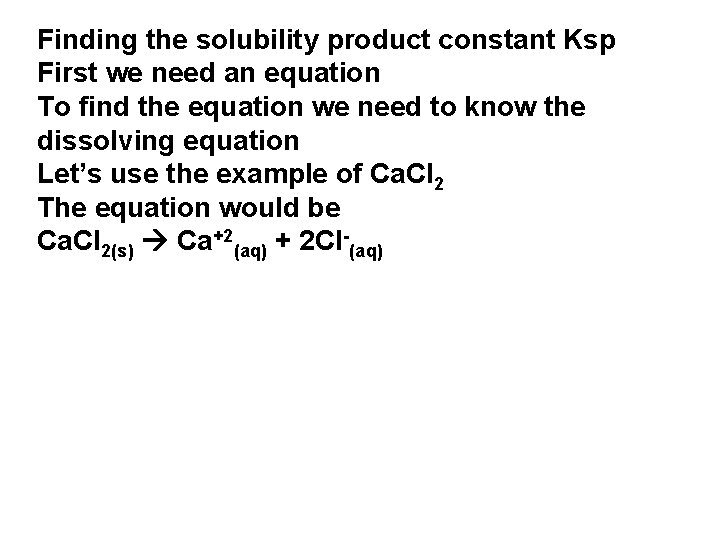
Finding the solubility product constant Ksp First we need an equation To find the equation we need to know the dissolving equation Let’s use the example of Ca. Cl 2 The equation would be Ca. Cl 2(s) Ca+2(aq) + 2 Cl-(aq)
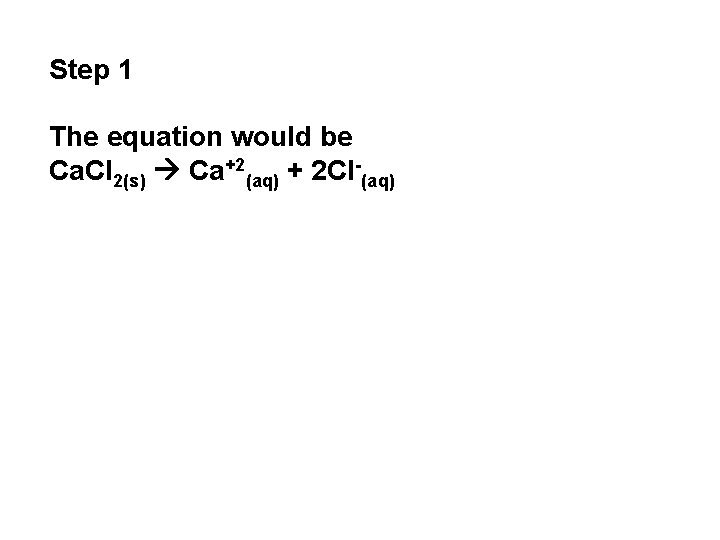
Step 1 The equation would be Ca. Cl 2(s) Ca+2(aq) + 2 Cl-(aq)
![Step 2 Constant = [Products] [Reactants] The equilibrium balance is not effected by how Step 2 Constant = [Products] [Reactants] The equilibrium balance is not effected by how](http://slidetodoc.com/presentation_image_h2/60eeb6d01968506772eeabe511c50786/image-7.jpg)
Step 2 Constant = [Products] [Reactants] The equilibrium balance is not effected by how much solid is left Ksp = [Ca+2]. [Cl-]2

Step 3 The equilibrium balance is not effected by how much solid is left Ksp = [Ca+2]. [Cl-]

Calculate the solubility product constant for Pb. Cl 2, if a saturated solution was found to contain 0. 1 M of Pb+2 and 0. 2 M of Cl. Step 1 write out ion equation Step 2 write out Ksp equation Don’t forget to raise each ion to the power of it’s coefficient Step 3 substitute in concentrations Step 4 solve

Step 1 write out ion/dissolving equation Pb. Cl 2 Pb+2 + 2 Cl- Step 2 write out Ksp equation using the steps Ksp = [Pb+2]. [Cl-]2
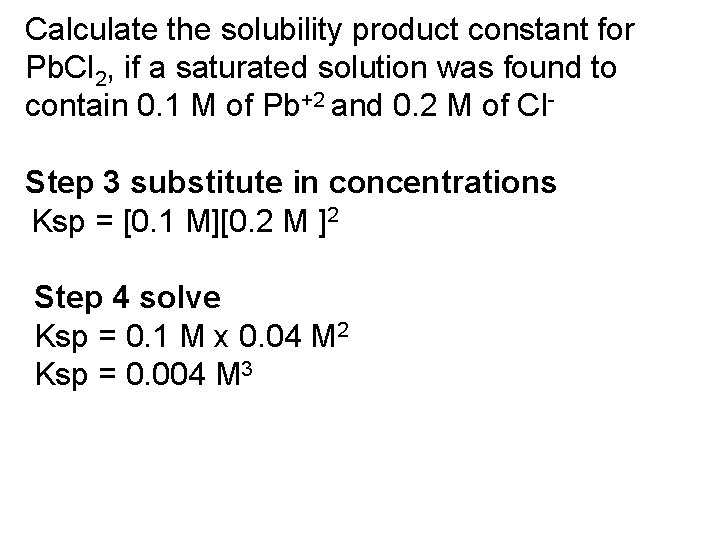
Calculate the solubility product constant for Pb. Cl 2, if a saturated solution was found to contain 0. 1 M of Pb+2 and 0. 2 M of Cl. Step 3 substitute in concentrations Ksp = [0. 1 M][0. 2 M ]2 Step 4 solve Ksp = 0. 1 M x 0. 04 M 2 Ksp = 0. 004 M 3

- Slides: 12