Ks from solubility data Calculate the solubility product

Ks from solubility data

Calculate the solubility product of Mn. CO 3, which has a solubility of 0. 0065 g per 100 m. L. M(Mn. CO 3) = 114. 9 g mol– 1 1 Convert the solubility into g L– 1 and then into mol L– 1.
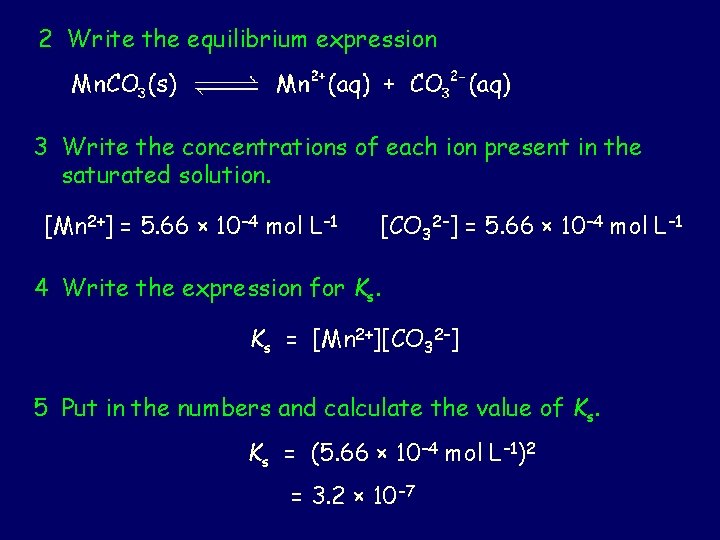
2 Write the equilibrium expression 3 Write the concentrations of each ion present in the saturated solution. [Mn 2+] = 5. 66 × 10– 4 mol L– 1 [CO 32–] = 5. 66 × 10– 4 mol L– 1 4 Write the expression for Ks. Ks = [Mn 2+][CO 32–] 5 Put in the numbers and calculate the value of Ks. Ks = (5. 66 × 10– 4 mol L– 1)2 = 3. 2 × 10 – 7

The solubility of Mg. F 2 is given as 0. 013 g per 100 m. L. Calculate the Ks. M(Mg. F 2) = 62. 3 g mol– 1. 1 Convert the solubility into g L– 1 and then into mol L– 1. Note: the final answer will be quoted to 2 sig fig, since that is the accuracy of the solubility data. Record the intermediary calculations to 3 or 4 sig fig though, and don’t clear the calculator between calculations, otherwise you introduce rounding errors at each step.

2 Write the equilibrium expression 3 Write the concentrations of each ion present in the saturated solution. [Mg 2+] = 2. 087 × 10– 3 mol L– 1 [F–] = 2 × 2. 087 × 10– 3 mol L– 1 = 4. 17 × 10– 3 mol L– 1 4 Write the expression for Ks. Ks = [Mg 2+][F–]2 5 Put in the numbers and calculate the value of Ks. Ks = (2. 087 × 10– 3 mol L– 1)(4. 17 × 10– 3 mol L– 1)2 = 3. 6 × 10– 8
- Slides: 5