KS 4 Chemistry Noble Gases 1 of 24
KS 4 Chemistry Noble Gases 1 of 24 © Boardworks Ltd 2005
Contents Noble Gases Discovery and electron structure Physical properties Uses Summary activities 2 of 24 © Boardworks Ltd 2005
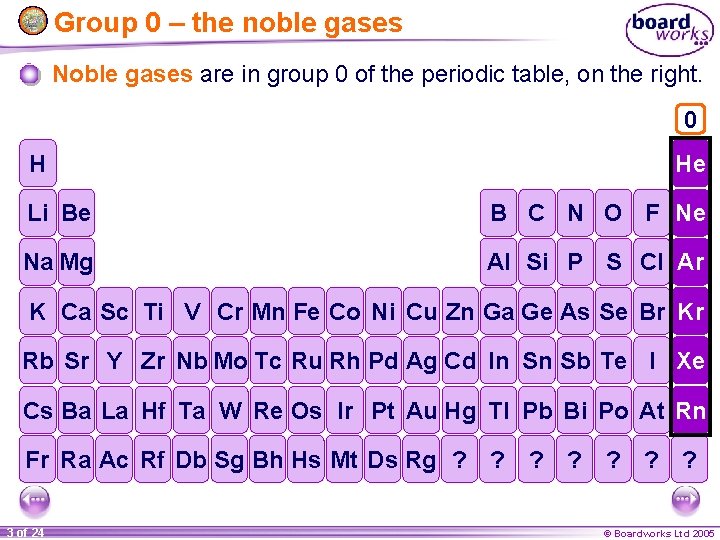
Group 0 – the noble gases Noble gases are in group 0 of the periodic table, on the right. 0 H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Ac Rf Db Sg Bh Hs Mt Ds Rg ? ? ? ? 3 of 24 © Boardworks Ltd 2005

4 of 24 © Boardworks Ltd 2005

Discovery of argon The noble gases were discovered and isolated in the 1890 s by William Ramsey, Lord Rayleigh, and Morris Travers. Noble gases had actually been first discovered, but not recognized, by Henry Cavendish in 1766. He had passed a series of electric sparks through a mixture of air and oxygen, and collected the gases that were produced. Each time he did the experiment, around 1% of the gas mixture did not react. Ramsay and his colleagues did further experiments and finally isolated a new element, which they called argon, from the Greek ‘argos’ meaning lazy or inactive. 5 of 24 © Boardworks Ltd 2005
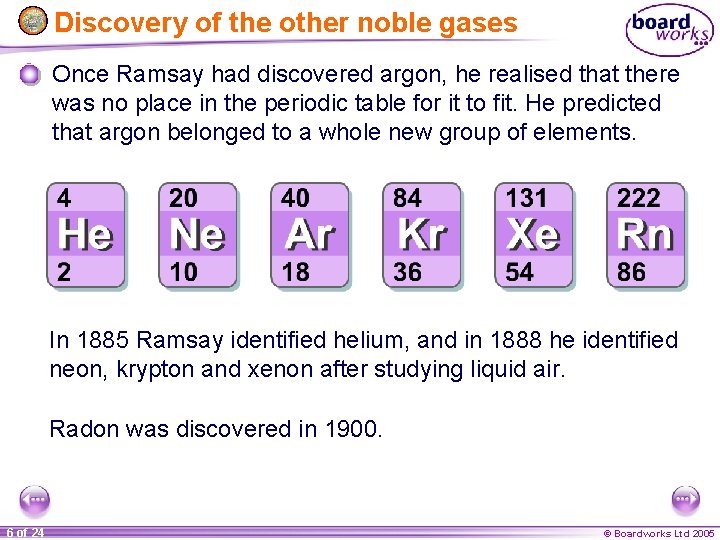
Discovery of the other noble gases Once Ramsay had discovered argon, he realised that there was no place in the periodic table for it to fit. He predicted that argon belonged to a whole new group of elements. In 1885 Ramsay identified helium, and in 1888 he identified neon, krypton and xenon after studying liquid air. Radon was discovered in 1900. 6 of 24 © Boardworks Ltd 2005

The noble gases Why are noble gases so unreactive? 7 of 24 © Boardworks Ltd 2005
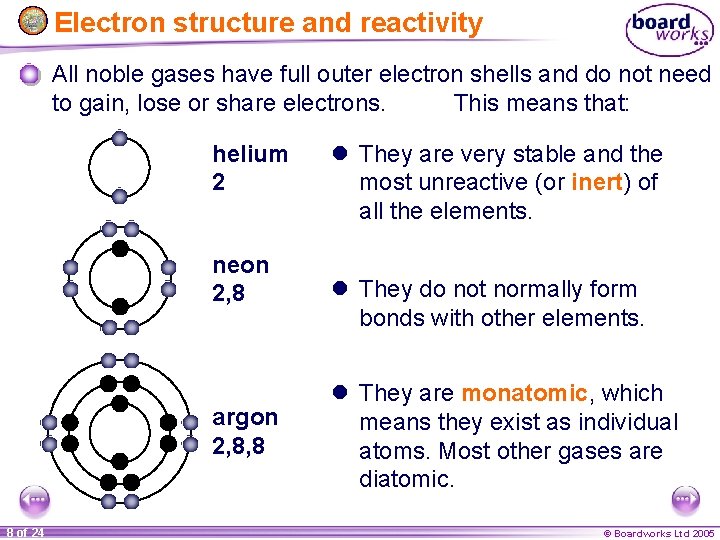
Electron structure and reactivity All noble gases have full outer electron shells and do not need to gain, lose or share electrons. This means that: helium 2 neon 2, 8 argon 2, 8, 8 8 of 24 l They are very stable and the most unreactive (or inert) of all the elements. l They do not normally form bonds with other elements. l They are monatomic, which means they exist as individual atoms. Most other gases are diatomic. © Boardworks Ltd 2005
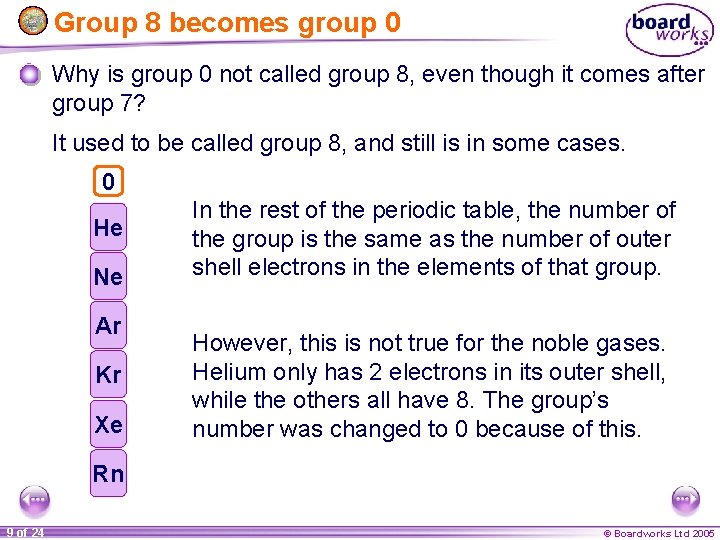
Group 8 becomes group 0 Why is group 0 not called group 8, even though it comes after group 7? It used to be called group 8, and still is in some cases. 8 0 He Ne Ar Kr Xe In the rest of the periodic table, the number of the group is the same as the number of outer shell electrons in the elements of that group. However, this is not true for the noble gases. Helium only has 2 electrons in its outer shell, while the others all have 8. The group’s number was changed to 0 because of this. Rn 9 of 24 © Boardworks Ltd 2005
Contents Noble Gases Discovery and electron structure Physical properties Uses Summary activities 10 of 24 © Boardworks Ltd 2005
General properties of noble gases All noble gases are colourless, odourless and unreactive. This makes them difficult to isolate and identify. Because noble gases are so unreactive, there are few patterns, or trends, among the group. 11 of 24 © Boardworks Ltd 2005
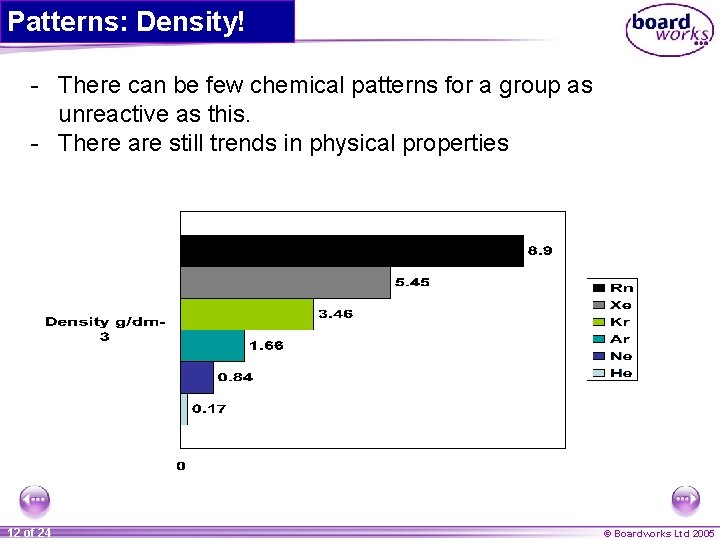
Patterns: Density! - There can be few chemical patterns for a group as unreactive as this. - There are still trends in physical properties 12 of 24 © Boardworks Ltd 2005
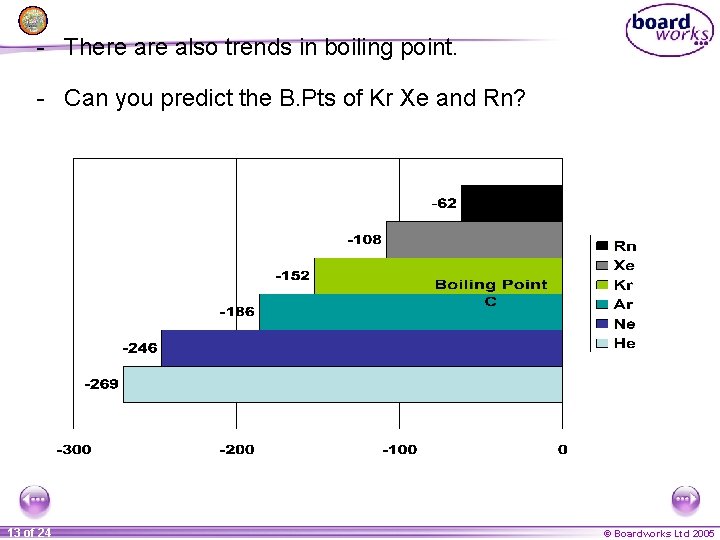
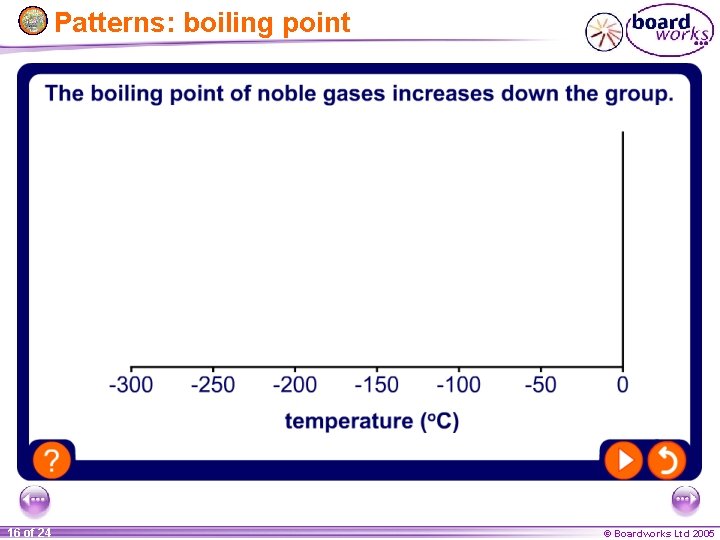
- There also trends in boiling point. - Can you predict the B. Pts of Kr Xe and Rn? 13 of 24 © Boardworks Ltd 2005
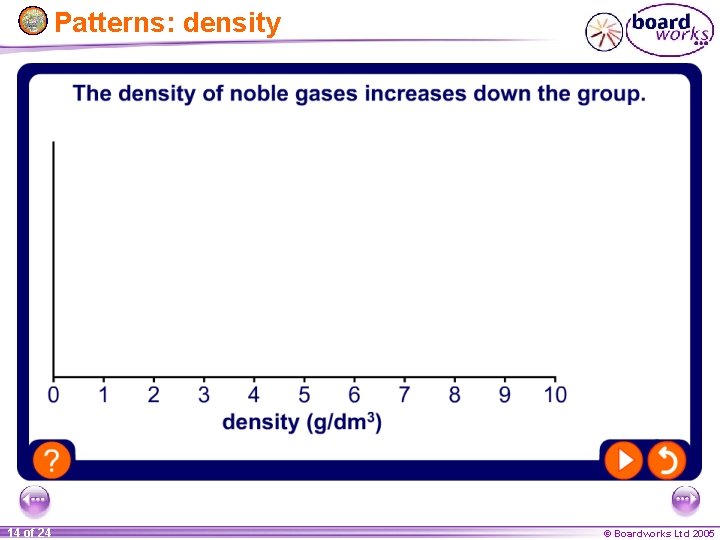
Patterns: density 14 of 24 © Boardworks Ltd 2005
Comparing the density of noble gases 15 of 24 © Boardworks Ltd 2005
Patterns: boiling point 16 of 24 © Boardworks Ltd 2005
Contents Noble Gases Discovery and electron structure Physical properties Uses Summary activities 17 of 24 © Boardworks Ltd 2005
Uses of noble gases Although noble gases are unreactive, they are still very useful elements. Many uses of noble gases depend on their ability to prevent other, undesirable, reactions taking place. 18 of 24 © Boardworks Ltd 2005

Uses of helium Helium is used as: l The gas for inflating balloons and airships, because it is less dense than air and inflammable. l A component of breathing gas (with oxygen) for deep-sea divers, because it is unreactive, insoluble and prevents divers getting ‘the bends’. l A protective gas for growing silicon crystals in silicon chip manufacture, because it is unreactive. l A super-coolant for high-performance magnets, e. g. in body scanners, because it has a very low boiling point (-269 °C). 19 of 24 © Boardworks Ltd 2005
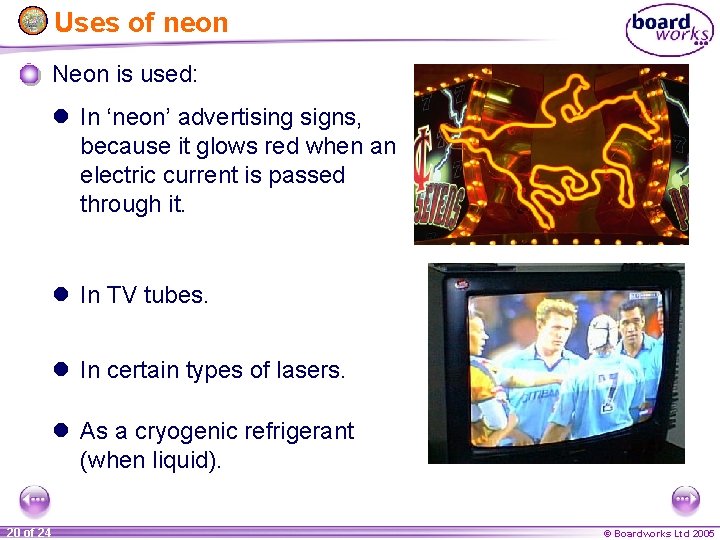
Uses of neon Neon is used: l In ‘neon’ advertising signs, because it glows red when an electric current is passed through it. l In TV tubes. l In certain types of lasers. l As a cryogenic refrigerant (when liquid). 20 of 24 © Boardworks Ltd 2005

Uses of argon Argon is used: l In normal wire-filament light bulbs, because it is unreactive and prevents the tungsten filament from burning. l In energy-efficient fluorescent light bulbs. l As a ‘gas blanket’ for arc welding, because it is unreactive and prevents the hot welding metal from oxidizing. 21 of 24 © Boardworks Ltd 2005

Uses of other noble gases Krypton is used: l In lasers for eye surgery, to stop bleeding on the retina. l In lighthouses and other types of lamps( airport landing lights) Xenon is used: l In various types of electron tubes, lamps , photographers flash gun and lasers. Radon is used: l To treat cancer by radiotherapy, because it is radioactive. However, because radon is radioactive, it is also an environmental hazard. 22 of 24 © Boardworks Ltd 2005
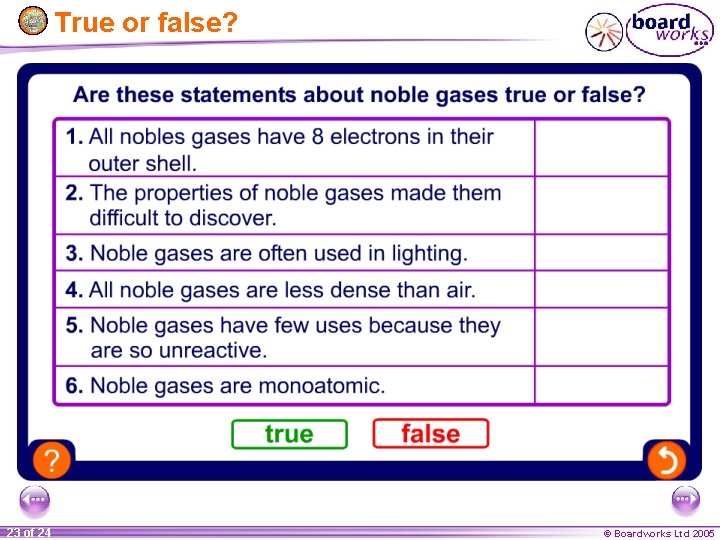
True or false? 23 of 24 © Boardworks Ltd 2005
Contents Noble Gases Discovery and electron structure Physical properties Uses Summary activities 24 of 24 © Boardworks Ltd 2005
Glossary l density – A measure of mass in a given volume. Often expressed in g/dm 3. l inert – Describes a substance that is unreactive under normal conditions. l monoatomic – An element that exists as a single atom. l noble gas – An element belonging to group 0 of the periodic table. l trend – A gradual change in a property or characteristic of elements in the same group of the periodic table. 25 of 24 © Boardworks Ltd 2005

Anagrams 26 of 24 © Boardworks Ltd 2005

Multiple-choice quiz 27 of 24 © Boardworks Ltd 2005
- Slides: 27