KS 3 Chemistry Solids Liquids and Gases 1





- Slides: 25

KS 3 Chemistry Solids, Liquids and Gases 1 of 25 20 © Boardworks Ltd 2005 2004

Contents 7 G Solids, Liquids and Gases Introducing states of matter The particle model Properties of solids, liquids and gases Diffusion Summary activities 1 20 2 of 25 © Boardworks Ltd 2005 2004

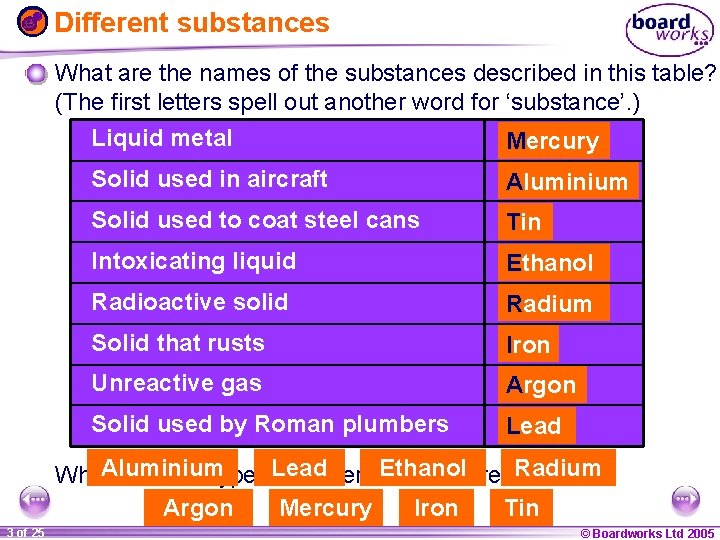
Different substances What are the names of the substances described in this table? (The first letters spell out another word for ‘substance’. ) Liquid metal Mercury Solid used in aircraft Aluminium Solid used to coat steel cans Tin Intoxicating liquid Ethanol Radioactive solid Radium Solid that rusts Iron Unreactive gas Argon Solid used by Roman plumbers Lead Ethanol What. Aluminium different types Lead of materials are there? Radium Argon Mercury Iron Tin 1 20 3 of 25 © Boardworks Ltd 2005 2004

Three states of matter At room temperature most substances exist in one of three physical states. solid 1 20 4 of 25 liquid gas © Boardworks Ltd 2005 2004

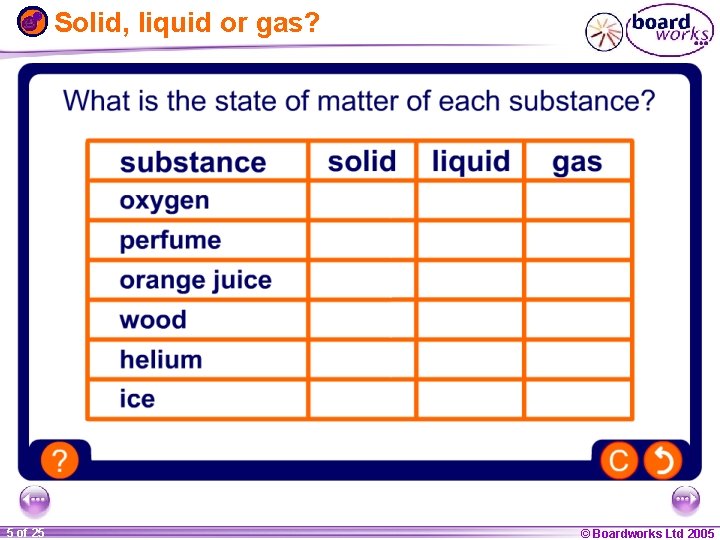
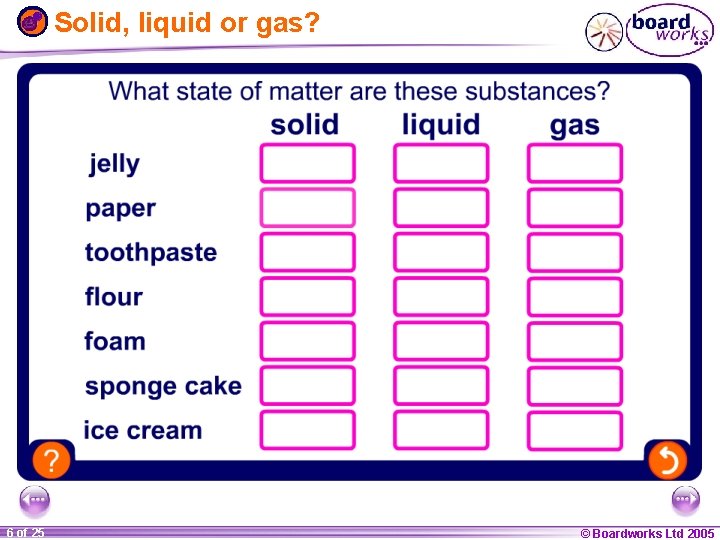
Solid, liquid or gas? 1 20 5 of 25 © Boardworks Ltd 2005 2004

Solid, liquid or gas? 1 20 6 of 25 © Boardworks Ltd 2005 2004

Contents 7 G Solids, Liquids and Gases Introducing states of matter The particle model Properties of solids, liquids and gases Diffusion Summary activities 1 20 7 of 25 © Boardworks Ltd 2005 2004

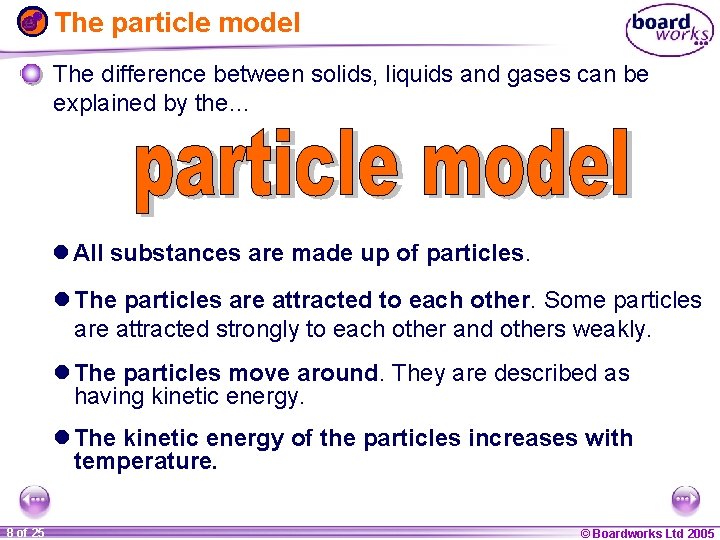
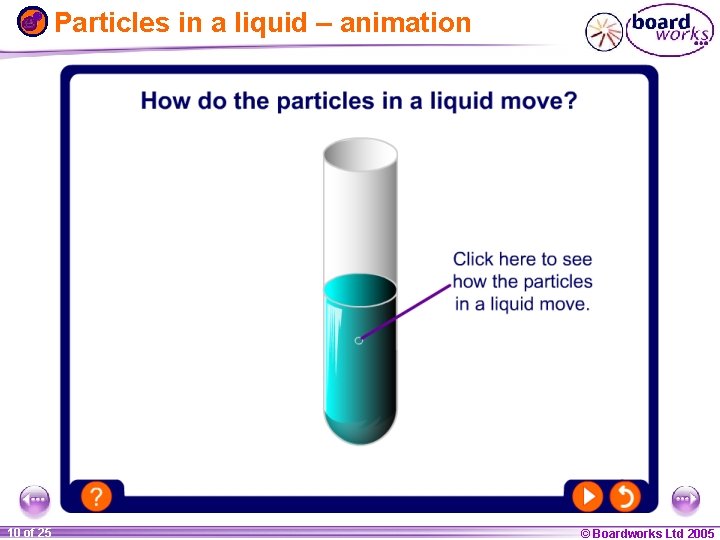
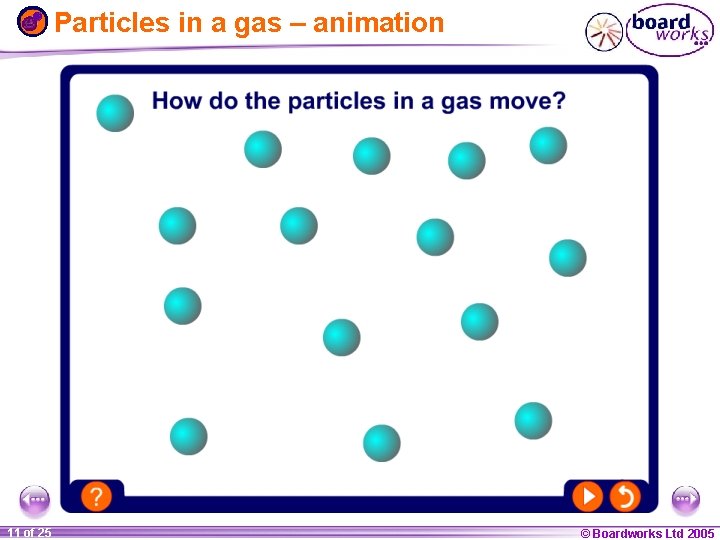
The particle model The difference between solids, liquids and gases can be explained by the… l All substances are made up of particles. l The particles are attracted to each other. Some particles are attracted strongly to each other and others weakly. l The particles move around. They are described as having kinetic energy. l The kinetic energy of the particles increases with temperature. 1 20 8 of 25 © Boardworks Ltd 2005 2004

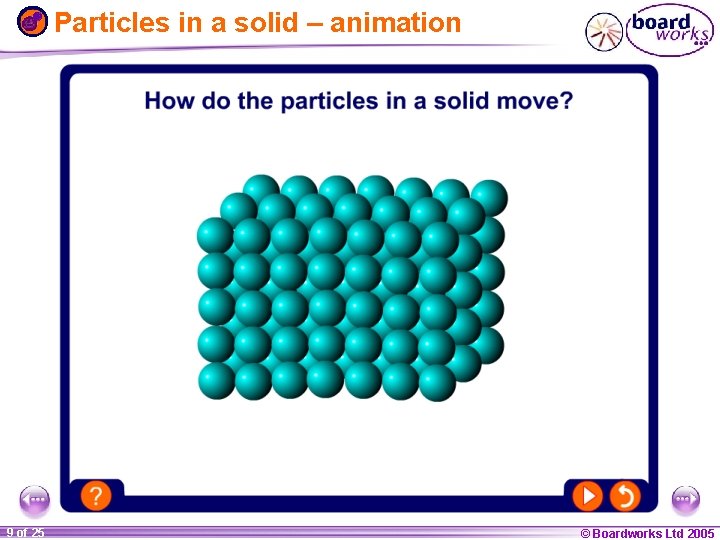
Particles in a solid – animation 1 20 9 of 25 © Boardworks Ltd 2005 2004

Particles in a liquid – animation 1 10 ofof 20 25 © Boardworks Ltd 2005 2004

Particles in a gas – animation 1 11 ofof 20 25 © Boardworks Ltd 2005 2004

Contents 7 G Solids, Liquids and Gases Introducing states of matter The particle model Properties of solids, liquids and gases Diffusion Summary activities 1 12 ofof 20 25 © Boardworks Ltd 2005 2004

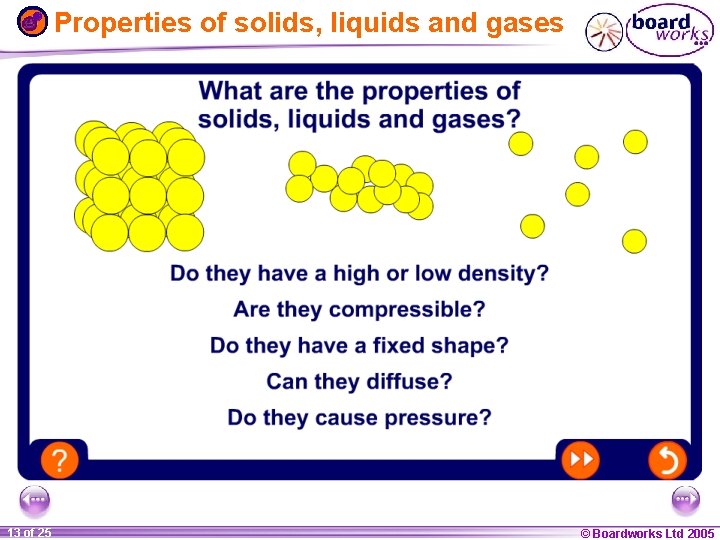
Properties of solids, liquids and gases 1 13 ofof 20 25 © Boardworks Ltd 2005 2004

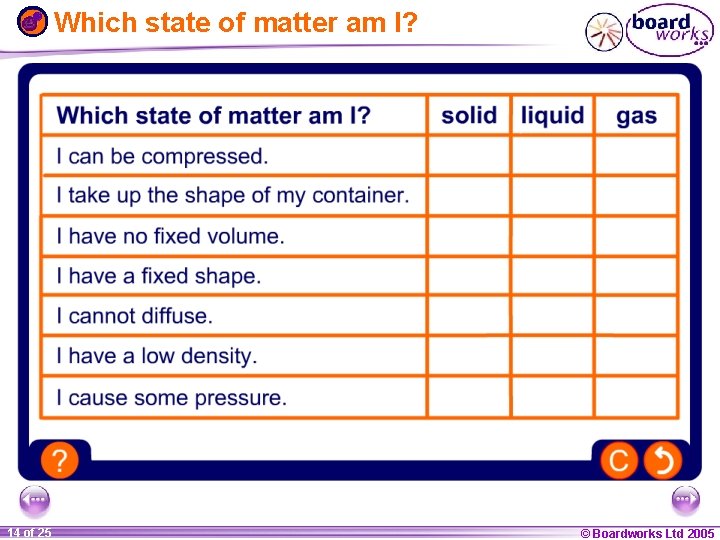
Which state of matter am I? 1 14 ofof 20 25 © Boardworks Ltd 2005 2004

Contents 7 G Solids, Liquids and Gases Introducing states of matter The particle model Properties of solids, liquids and gases Diffusion Summary activities 1 15 ofof 20 25 © Boardworks Ltd 2005 2004

How do smells spread out? Where is the smell coming from and how does it spread out? 1 16 ofof 20 25 © Boardworks Ltd 2005 2004

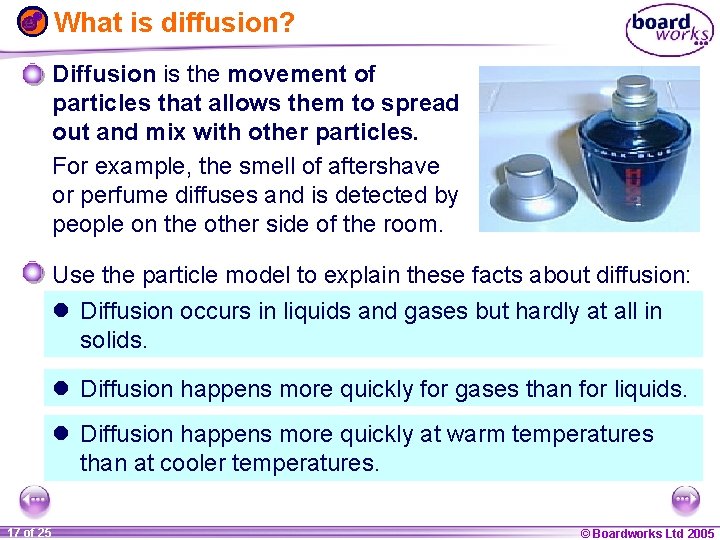
What is diffusion? Diffusion is the movement of particles that allows them to spread out and mix with other particles. For example, the smell of aftershave or perfume diffuses and is detected by people on the other side of the room. Use the particle model to explain these facts about diffusion: l Diffusion occurs in liquids and gases but hardly at all in solids. l Diffusion happens more quickly for gases than for liquids. l Diffusion happens more quickly at warm temperatures than at cooler temperatures. 1 17 ofof 20 25 © Boardworks Ltd 2005 2004

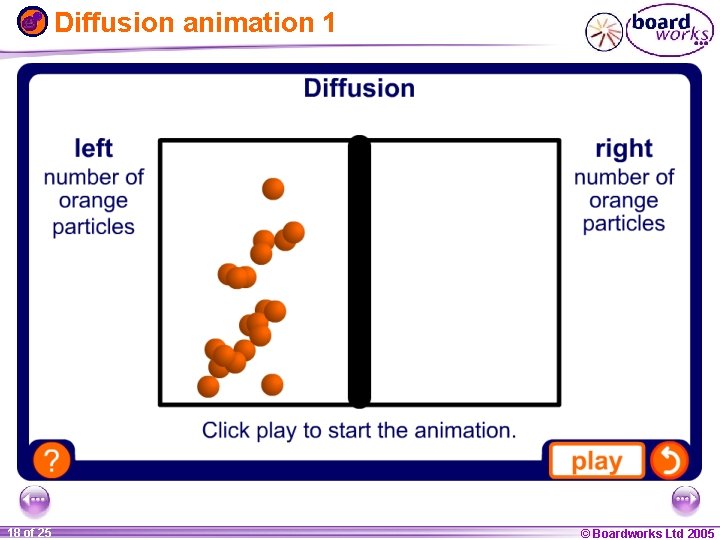
Diffusion animation 1 1 18 ofof 20 25 © Boardworks Ltd 2005 2004

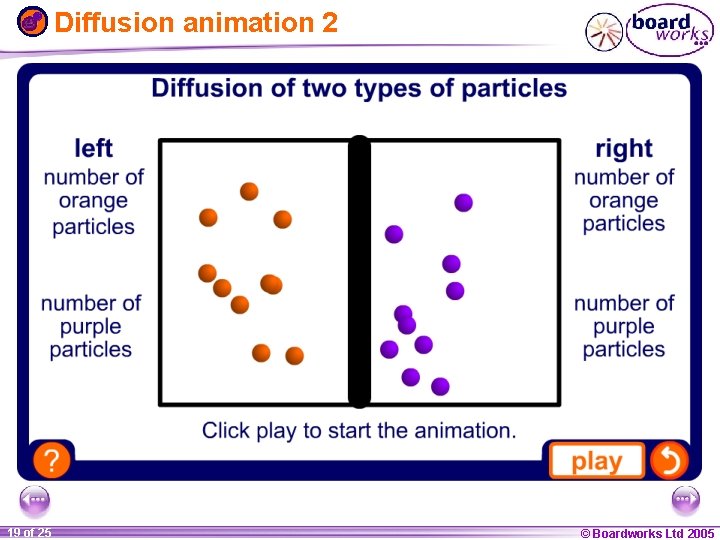
Diffusion animation 2 1 19 ofof 20 25 © Boardworks Ltd 2005 2004

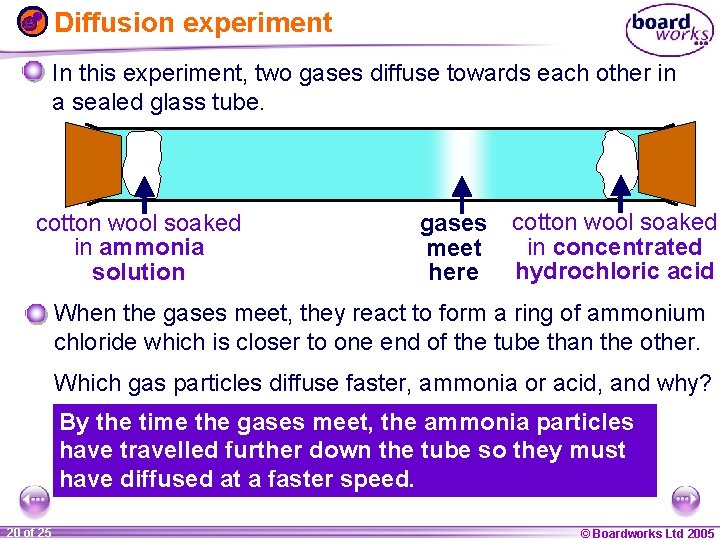
Diffusion experiment In this experiment, two gases diffuse towards each other in a sealed glass tube. cotton wool soaked in ammonia solution gases meet here cotton wool soaked in concentrated hydrochloric acid When the gases meet, they react to form a ring of ammonium chloride which is closer to one end of the tube than the other. Which gas particles diffuse faster, ammonia or acid, and why? By the time the gases meet, the ammonia particles have travelled further down the tube so they must have diffused at a faster speed. 1 20 ofof 20 25 © Boardworks Ltd 2005 2004

Contents 7 G Solids, Liquids and Gases Introducing states of matter The particle model Properties of solids, liquids and gases Diffusion Summary activities 1 21 ofof 20 25 © Boardworks Ltd 2005 2004

Glossary diffusion – Particles spreading out and mixing in the gas or liquid state. gas – The state of matter in which particles move quickly in all directions and rarely touch each other. liquid – The state of matter in which particles are randomly arranged and touch each other. matter – The stuff that everything is made of. particle – The smallest unit of matter. pressure – The force produced when particles move against a surface. solid – The state of matter in which particles are in a fixed arrangement and touch each other. 1 22 ofof 20 25 © Boardworks Ltd 2005 2004

Anagrams 1 23 ofof 20 25 © Boardworks Ltd 2005 2004

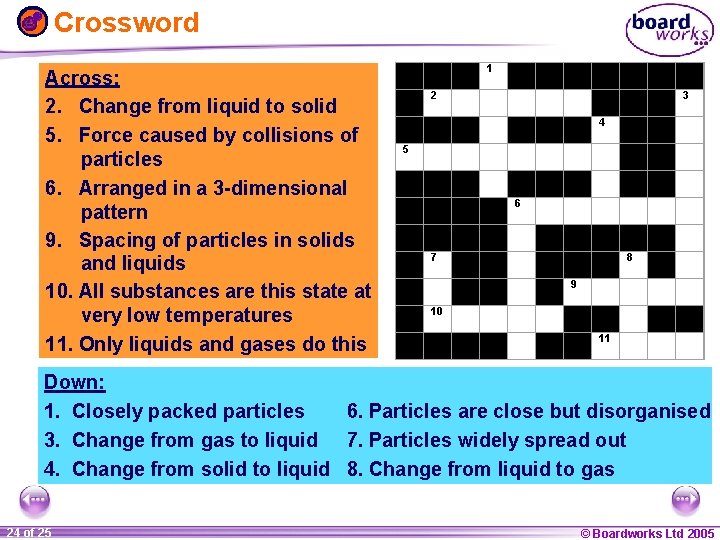
Crossword Across: 2. Change from liquid to solid 5. Force caused by collisions of particles 6. Arranged in a 3 -dimensional pattern 9. Spacing of particles in solids and liquids 10. All substances are this state at very low temperatures 11. Only liquids and gases do this 1 2 3 4 5 6 7 8 9 10 11 Down: 1. Closely packed particles 6. Particles are close but disorganised 3. Change from gas to liquid 7. Particles widely spread out 4. Change from solid to liquid 8. Change from liquid to gas 1 24 ofof 20 25 © Boardworks Ltd 2005 2004

Multiple-choice quiz 1 25 ofof 20 25 © Boardworks Ltd 2005 2004