KP 1 What is heat In science heat







- Slides: 11

KP 1: What is heat? • In science, heat is a type of energy • All objects are made of small, constantly moving particles – Heat energy measures the total energy of all of these moving particles • The more energy in the object, the more heat it has

KP 2: What is the difference between heat and temperature? • A lot of people think heat and temperature are the same thing, but this is not true! – Temperature is the average energy of all particles – Heat is the total energy of all particles

• Example: what has a higher temperature: a pot of hot soup or the Pacific Ocean? • Example: what has more heat energy: a pot of hot soup or the Pacific Ocean?

KP 3: How do we measure temperature? • Temperature is measured using something called a thermometer • Thermometers work by something called heat expansion – When objects get hot, they move faster, and when they move faster, they expand

DEMO

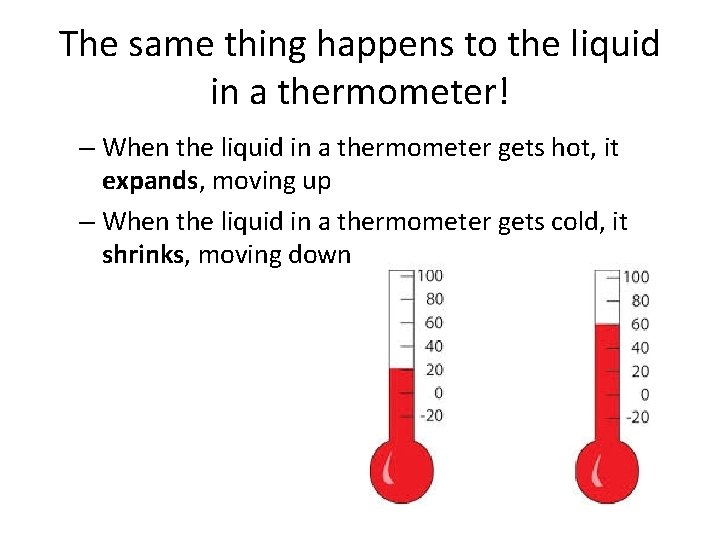
The same thing happens to the liquid in a thermometer! – When the liquid in a thermometer gets hot, it expands, moving up – When the liquid in a thermometer gets cold, it shrinks, moving down

KP 4: What are the different temperature scales? • In science, we use 2 temperature scales: celsius (C) and kelvin (K) • 0 degrees Kelvin = -273 degrees Celsius – This means to change C to K, you can add 273 – To change K to C, you can subtract 273 Example: How many degrees K is 50 degrees C?

• Example: How many degrees C is 400 degrees K? • Example: How many degrees K is 20 degrees C?

Guided Practice 1. What has a higher temperature: a teaspoon of boiling water or an iceberg? Why?

2. What has more heat energy: a teaspoon of boiling water or an iceberg? Why?

3. Convert between the following units: a. How many degrees K is 30 degrees C? b. b. How many degrees C is 150 degrees K?