Komperda ORGANIZATION OF THE PERIODIC TABLE Komperda Questions

Komperda ORGANIZATION OF THE PERIODIC TABLE

Komperda Questions: Mendeleev Reading What is a period? What is a group? List the ways the Periodic Table is organized:
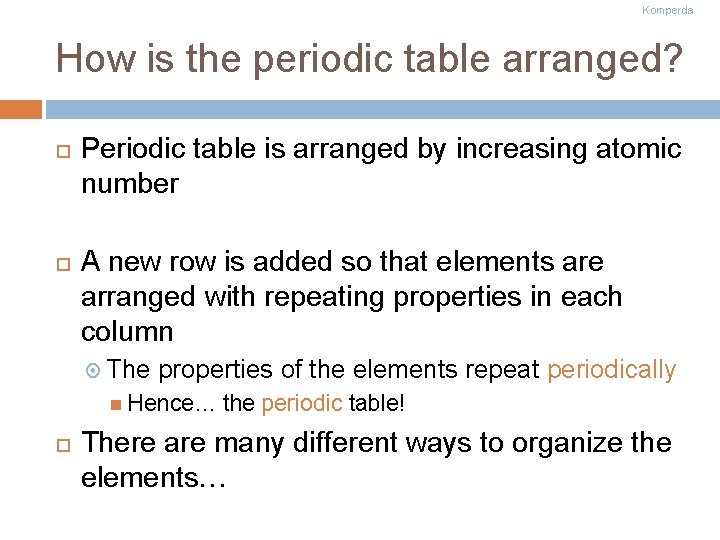
Komperda How is the periodic table arranged? Periodic table is arranged by increasing atomic number A new row is added so that elements are arranged with repeating properties in each column The properties of the elements repeat periodically Hence… the periodic table! There are many different ways to organize the elements…
Komperda Circular
Komperda Long Form
Komperda Spiral
Komperda Pyramid
Komperda Layers
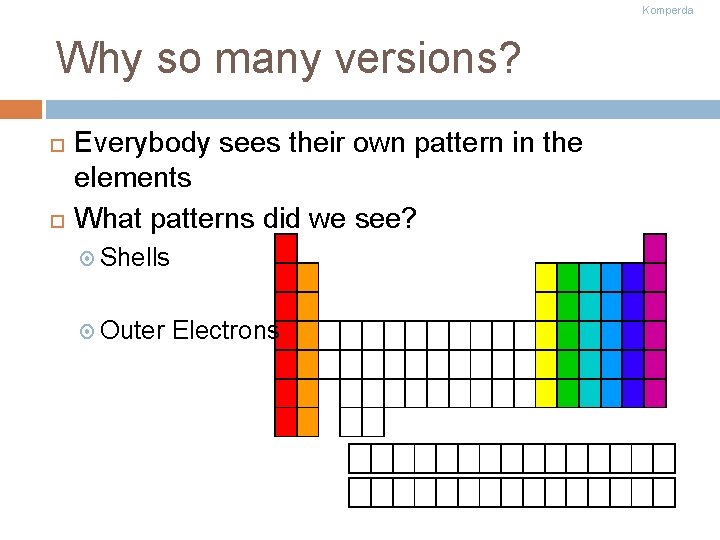
Komperda Why so many versions? Everybody sees their own pattern in the elements What patterns did we see? Shells Outer Electrons

Komperda Dmitri Mendeleyev Father of the Modern P. T.
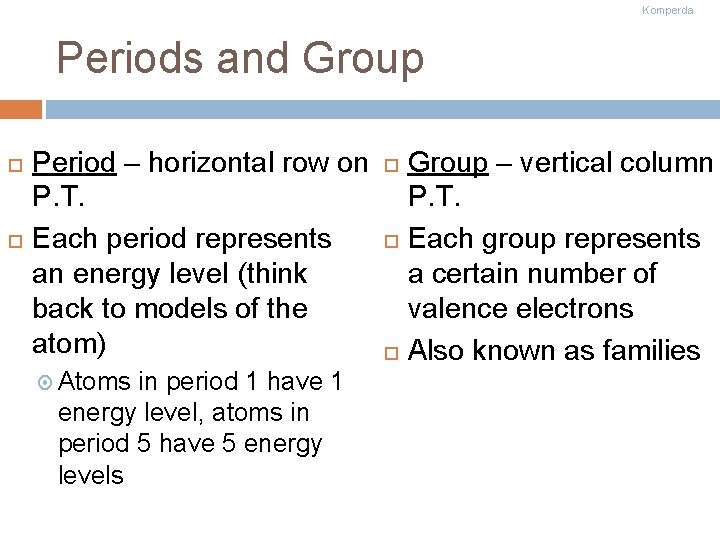
Komperda Periods and Group Period – horizontal row on P. T. Each period represents an energy level (think back to models of the atom) Atoms in period 1 have 1 energy level, atoms in period 5 have 5 energy levels Group – vertical column P. T. Each group represents a certain number of valence electrons Also known as families
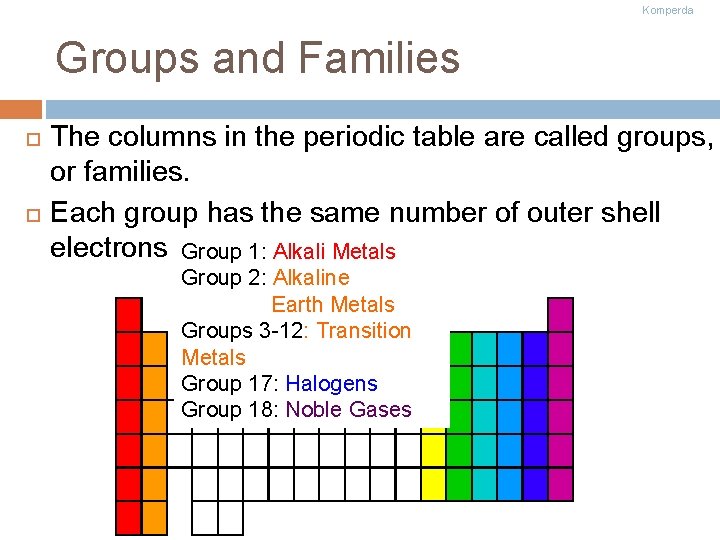
Komperda Groups and Families The columns in the periodic table are called groups, or families. Each group has the same number of outer shell electrons Group 1: Alkali Metals Group 2: Alkaline Earth Metals Groups 3 -12: Transition Metals Group 17: Halogens Group 18: Noble Gases
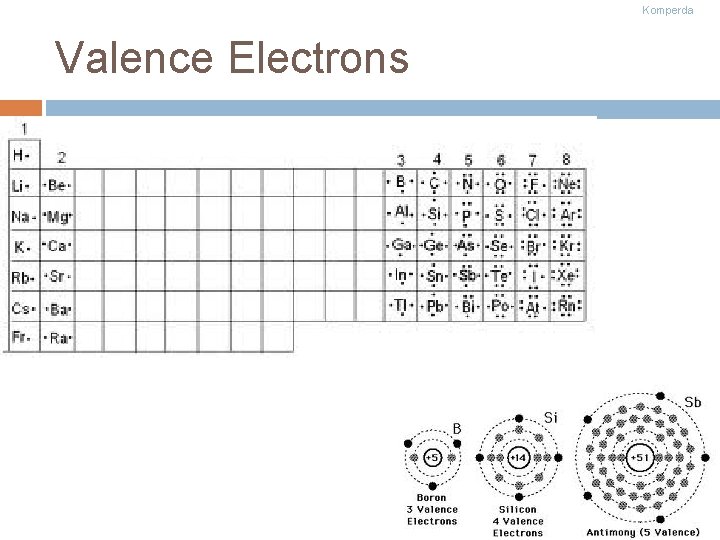
Komperda Valence Electrons exist within energy levels. The electrons in the outermost energy level are called valence electrons! Valence electrons determine how an atom behaves (they perform all bonding)

Komperda Valence Electrons Ctd Each group number represents the number of valence electrons elements in that group have Ex: group 1 has 1 valence electron No atom can have more than 8 v. e. For group numbers 13 - 18 subtract ten to figure the number of valence electrons Ex: Group 18 = 8 valence electrons Groups 3 -12, we assume to have 2 v. e. (not always the case)
Komperda Valence Electrons

Komperda 8 is great (sometimes 2) The noble gases (group 18) are stable atoms, meaning they do not react! (this is good) The noble gases are stable because they have 8 valence electrons (or 2 as in helium) All other atoms will gain or lose electrons to become like noble gases (remember cations and anions)
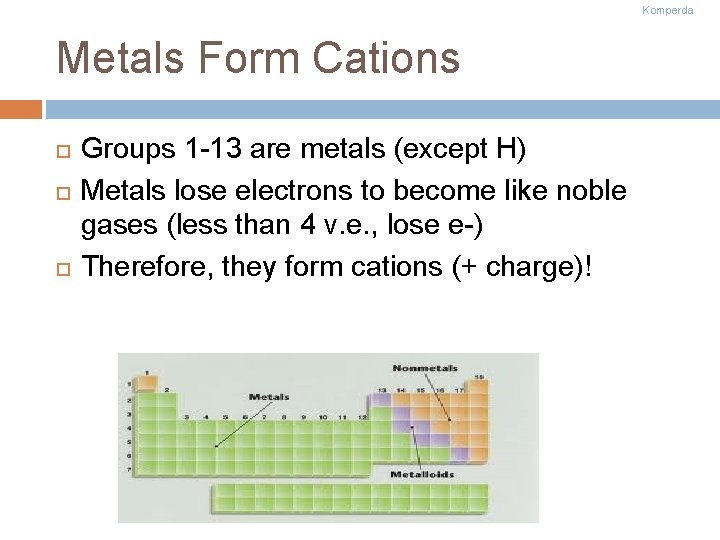
Komperda Metals Form Cations Groups 1 -13 are metals (except H) Metals lose electrons to become like noble gases (less than 4 v. e. , lose e-) Therefore, they form cations (+ charge)!
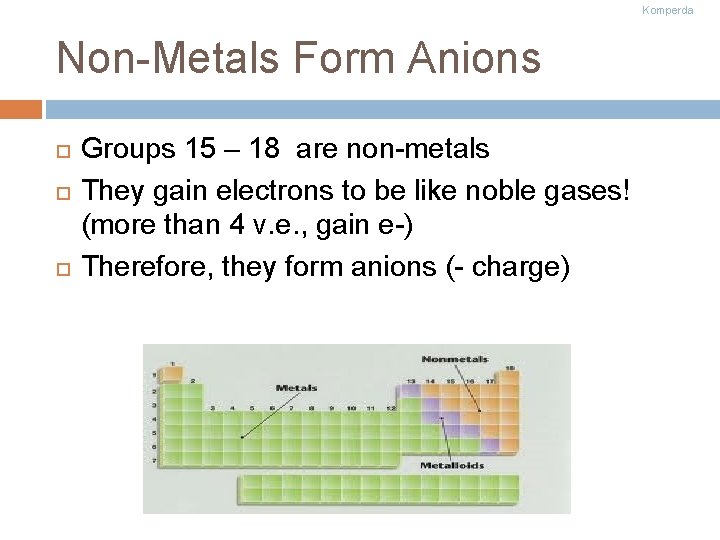
Komperda Non-Metals Form Anions Groups 15 – 18 are non-metals They gain electrons to be like noble gases! (more than 4 v. e. , gain e-) Therefore, they form anions (- charge)

Komperda Practice For each of the following elements, determine if they are a metal or non-metal and the charge they would form. Na Al S O F Ba Cs I P
Komperda Typical Charge (Ion Formed) You should remember that 8 is great! Elements will either gain or lose electrons to try to have 8 Elements with 3 or less valence electrons will LOSE electrons Becoming _______ Elements with 4 or more valence electrons will GAIN electrons Becoming _______ For Hydrogen and Helium, 2 is good too! They at 2 can’t possibly hold 8 electrons, so they are full
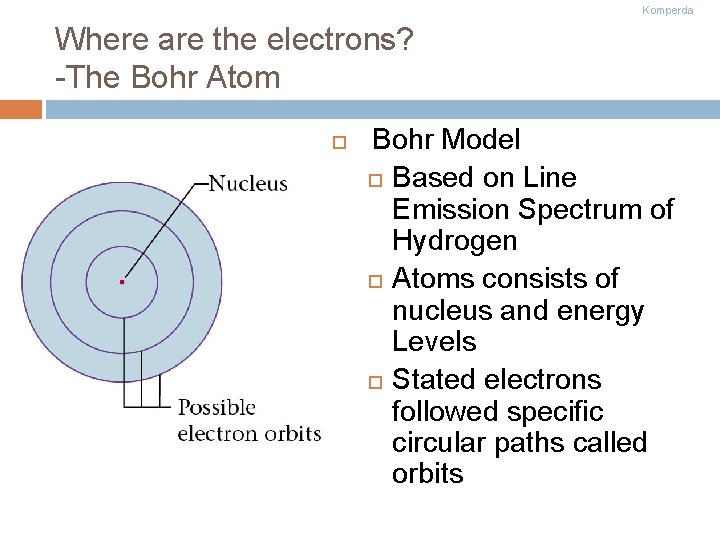
Komperda Where are the electrons? -The Bohr Atom Bohr Model Based on Line Emission Spectrum of Hydrogen Atoms consists of nucleus and energy Levels Stated electrons followed specific circular paths called orbits
Komperda Quantum Mechanical Model Consists of Energy levels, sublevels, and orbitals Key Points: 1. Electrons do not follow orbits, nor can location be known exactly 2. Electrons are located within orbitals (probable location of electron)
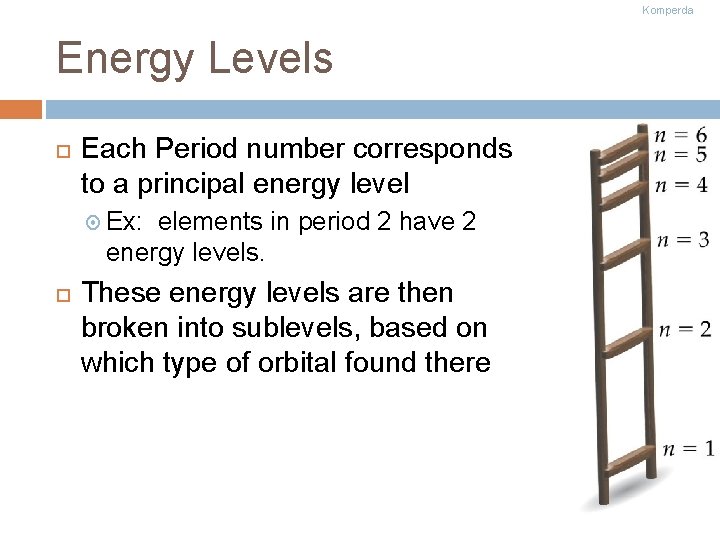
Komperda Energy Levels Each Period number corresponds to a principal energy level Ex: elements in period 2 have 2 energy levels. These energy levels are then broken into sublevels, based on which type of orbital found there
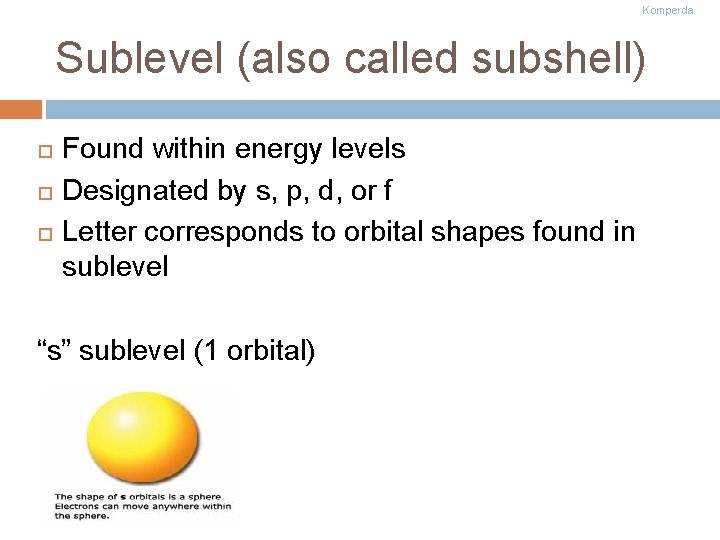
Komperda Sublevel (also called subshell) Found within energy levels Designated by s, p, d, or f Letter corresponds to orbital shapes found in sublevel “s” sublevel (1 orbital)
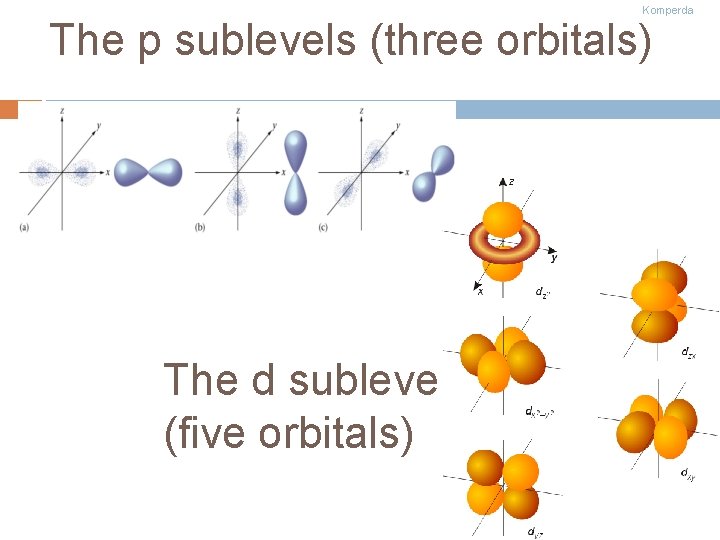
Komperda The p sublevels (three orbitals) The d sublevel (five orbitals)

Komperda The f sublevel (7 orbitals)
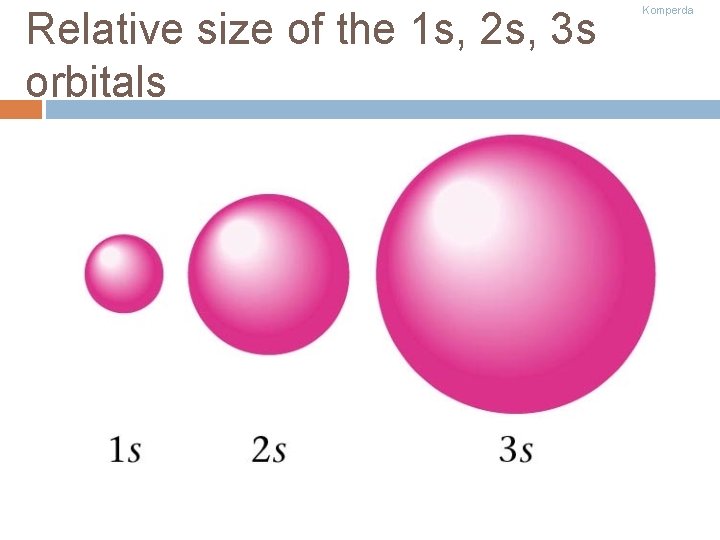
Relative size of the 1 s, 2 s, 3 s orbitals Komperda

Komperda Orbitals Generalized location of electron You know I’m probably in this room all day, you just don’t know if I’m at my desk or in the storeroom or walking around Does not have sharp edges 1 orbital can contain a maximum of 2 electrons
Komperda Electron Configuration Electron configuration: description of what sublevels and orbitals are filled by electrons in any given atom (like a roadmap of the electrons in an atom) Determined by the number of electrons the atom has Governed by 3 rules!

Komperda e- configuration rules Aufbau Principle: an electron occupies the lowest energy level & orbital available Pauli Exclusion Principle: only two electrons can occupy any orbital, and they must have opposite spins Hund’s Rule: Each orbital in a given sublevel (s, p, d, or f orbital) must have 1 electron before any can have two
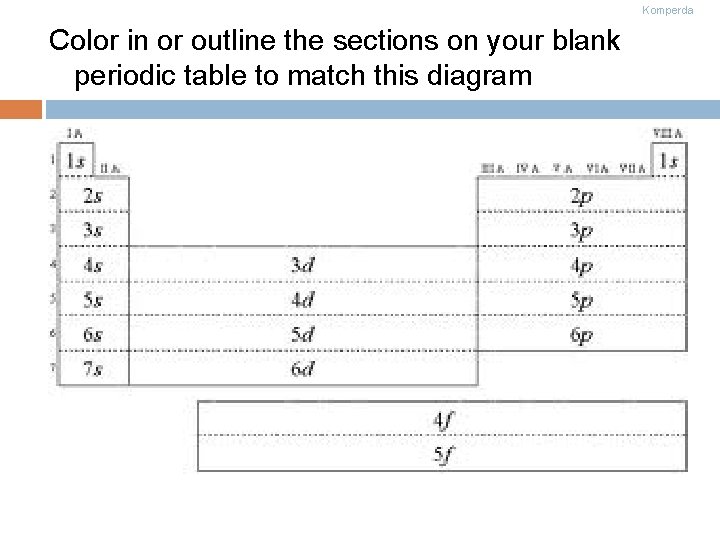
Komperda Color in or outline the sections on your blank periodic table to match this diagram

Komperda Orbital Diagrams Using the periodic table from the previous slide, we can start to understand how electron configurations work. (example below) Ex: Write the Orbital Diagram Magnesium. 1: Determine the atomic number of the element from the Periodic Table This gives the number of protons and electrons in a neutral atom of that element Mg, Z = 12, so Mg has 12 protons and 12 electrons
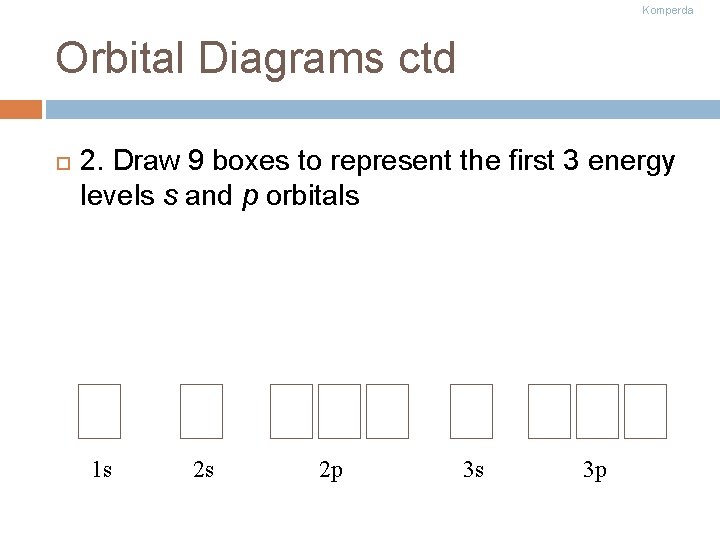
Komperda Orbital Diagrams ctd 2. Draw 9 boxes to represent the first 3 energy levels s and p orbitals 1 s 2 s 2 p 3 s 3 p
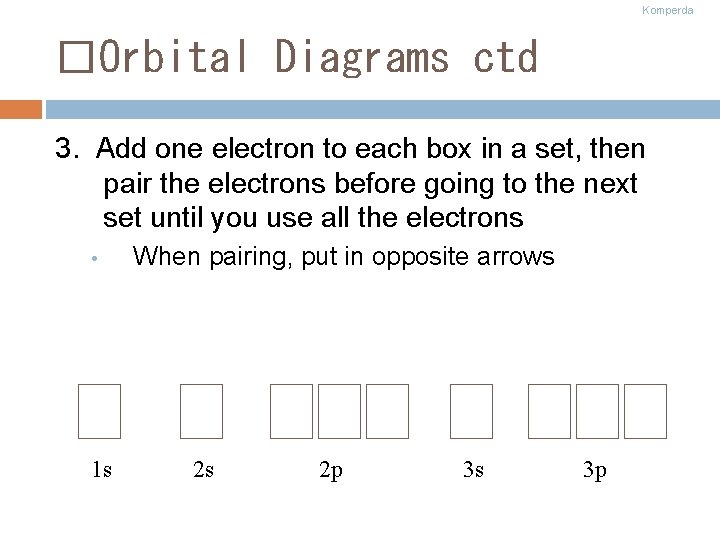
Komperda �Orbital Diagrams ctd 3. Add one electron to each box in a set, then pair the electrons before going to the next set until you use all the electrons • 1 s When pairing, put in opposite arrows 2 s 2 p 3 s 3 p
Komperda Orbital Diagram Practice Write orbital diagrams for each of the following elements: Ne Be Al
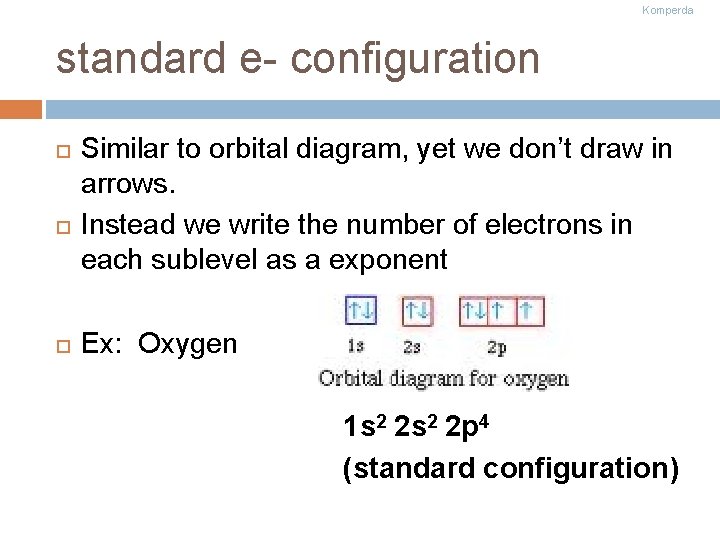
Komperda standard e- configuration Similar to orbital diagram, yet we don’t draw in arrows. Instead we write the number of electrons in each sublevel as a exponent Ex: Oxygen 1 s 2 2 p 4 (standard configuration)
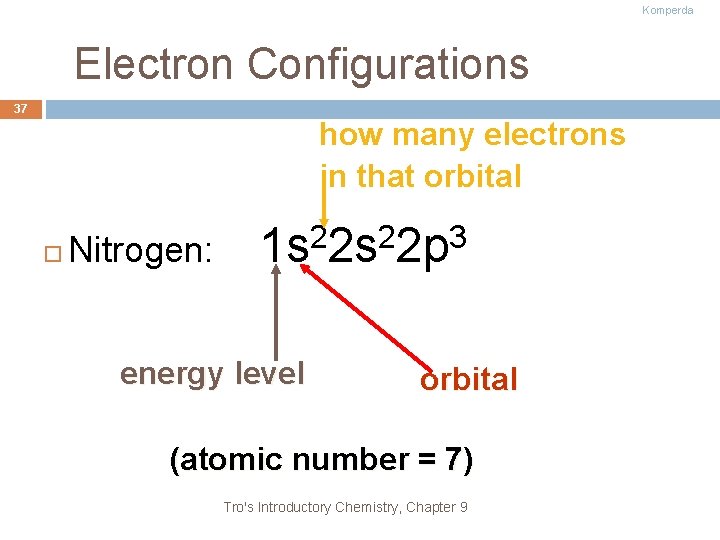
Komperda Electron Configurations 37 how many electrons in that orbital Nitrogen: 2 2 3 1 s 2 s 2 p energy level orbital (atomic number = 7) Tro's Introductory Chemistry, Chapter 9
Standard e- configuration practice For each of the following, write out the standard electron configuration: Mg Ne S Fe Komperda
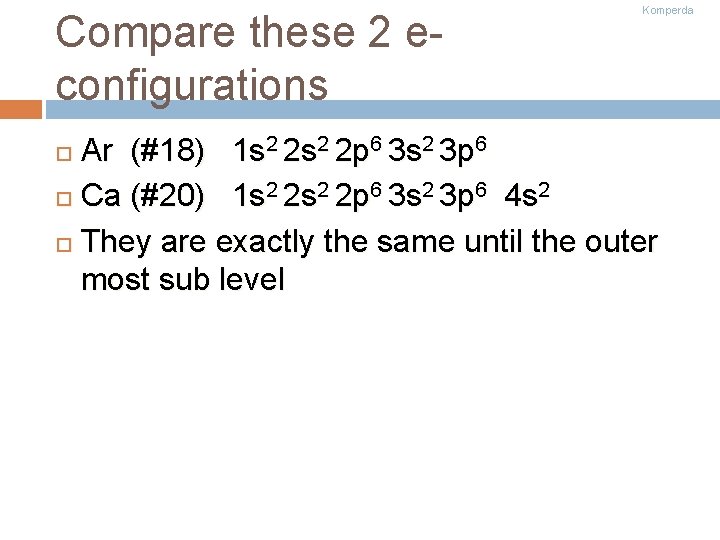
Compare these 2 econfigurations Komperda Ar (#18) 1 s 2 2 p 6 3 s 2 3 p 6 Ca (#20) 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 They are exactly the same until the outer most sub level

Noble Gas (abbreviated) configuration Komperda We use the elements in group 18, the ones with a full octet, the noble gases to make a short cut. (remember they do not gain or lose e-) [Ar] 4 s 2 tells us that Ca has the same econfiguration as Ar, except for the valence electrons, which are in 4 s 2.

Noble Gas (abbreviated) practice For each of the following, list the Noble Gas configuration Li Pb Fe I Kr Komperda

Komperda Exceptions to Rules Groups 6 & 11 do not follow all rules exactly! They steal one electron from the previous “s” sublevel to stabilize their unfilled “d” orbital Ex:

Komperda Charges & E- Config Determine number of electrons and write e-config to go along with number of e. Metals form Cations (lose electrons) Na+1 Ca+2 Non-Metals form Anions (gain electrons) F-1 S-2

Komperda Quiz next class (Fri 11/16) Ions (how to determine charge) Three rules governing E config Aufbau Pauli Hunds Three types of e config Orbital Standard Abbreviated (noble gas) E config for Ions/Exceptions
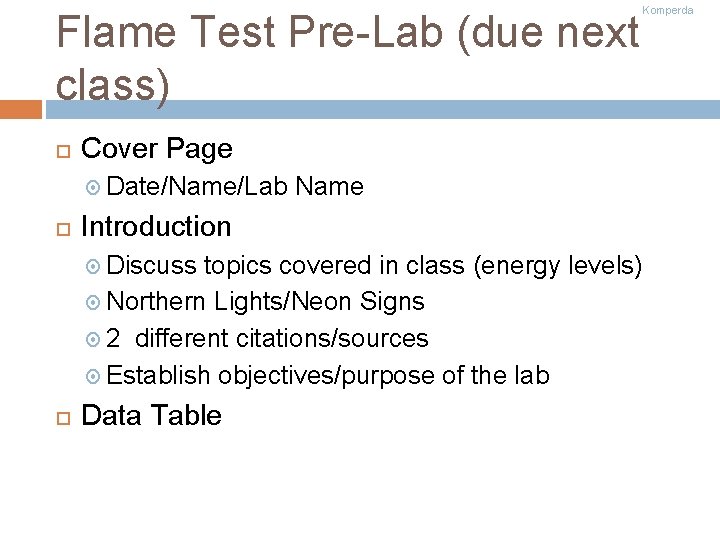
Flame Test Pre-Lab (due next class) Cover Page Date/Name/Lab Name Introduction Discuss topics covered in class (energy levels) Northern Lights/Neon Signs 2 different citations/sources Establish objectives/purpose of the lab Data Table Komperda

Flame Test – Final Report (due 11/20) Title Page Intro Data Table Post Lab Questions (just 1 -5) Type Komperda Reponses in complete sentences Conclusion What was learned (examples of flame colors for ions)? 2 Possible Errors and Impact on observations Identification of unknown with references to data table to support
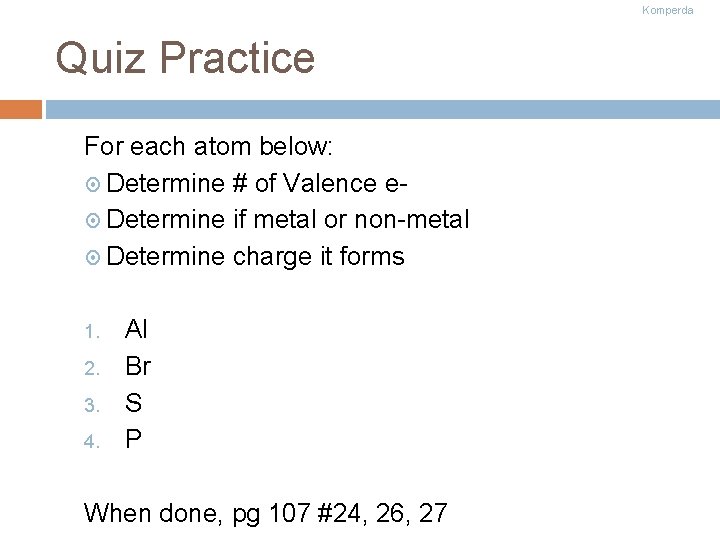
Komperda Quiz Practice For each atom below: Determine # of Valence e Determine if metal or non-metal Determine charge it forms 1. 2. 3. 4. Al Br S P When done, pg 107 #24, 26, 27
- Slides: 47