Knowledge Organiser The Periodic Table Year 9 Structure
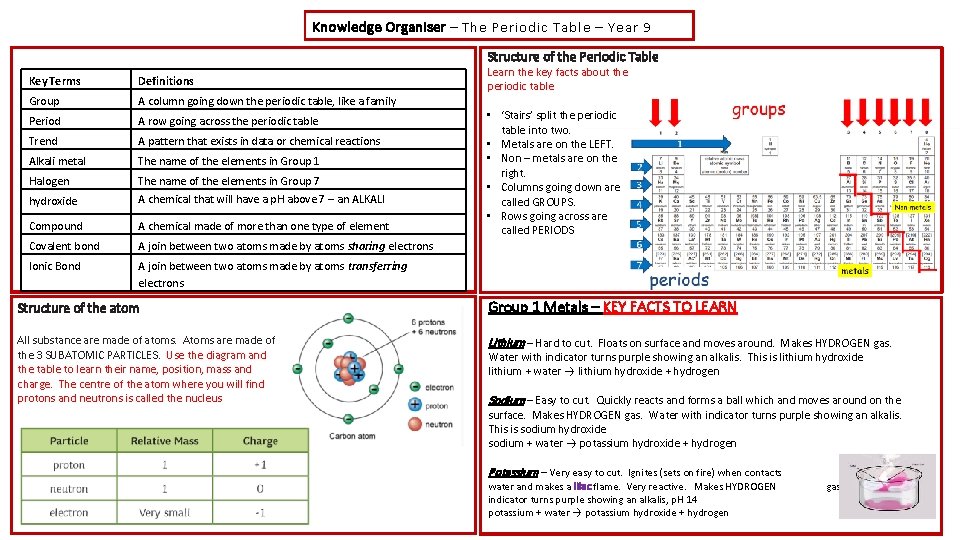
Knowledge Organiser – The Periodic Table – Year 9 Structure of the Periodic Table Key Terms Definitions Group A column going down the periodic table, like a family Period A row going across the periodic table Trend A pattern that exists in data or chemical reactions Alkali metal The name of the elements in Group 1 Halogen hydroxide The name of the elements in Group 7 A chemical that will have a p. H above 7 – an ALKALI Compound A chemical made of more than one type of element Covalent bond A join between two atoms made by atoms sharing electrons Ionic Bond A join between two atoms made by atoms transferring electrons Learn the key facts about the periodic table • ‘Stairs’ split the periodic table into two. • Metals are on the LEFT. • Non – metals are on the right. • Columns going down are called GROUPS. • Rows going across are called PERIODS Structure of the atom Group 1 Metals – KEY FACTS TO LEARN All substance are made of atoms. Atoms are made of the 3 SUBATOMIC PARTICLES. Use the diagram and the table to learn their name, position, mass and charge. The centre of the atom where you will find protons and neutrons is called the nucleus Lithium – Hard to cut. Floats on surface and moves around. Makes HYDROGEN gas. Water with indicator turns purple showing an alkalis. This is lithium hydroxide lithium + water → lithium hydroxide + hydrogen Sodium – Easy to cut. Quickly reacts and forms a ball which and moves around on the surface. Makes HYDROGEN gas. Water with indicator turns purple showing an alkalis. This is sodium hydroxide sodium + water → potassium hydroxide + hydrogen Potassium – Very easy to cut. Ignites (sets on fire) when contacts water and makes a lilac flame. Very reactive. Makes HYDROGEN indicator turns purple showing an alkalis, p. H 14 potassium + water → potassium hydroxide + hydrogen with gas. Water with

Knowledge Organiser – The Periodic Table – Year 9 Group 7 – The halogens – KEY FACTS TO LEARN • • All of the halogens are non-metals • They do not conduct heat or electricity • The halogens exist as simple molecules – eg F 2 • The melting points and boiling points of the halogens increase going down A more reactive halogen can displace a less reactive halogen from a compounds group 7. • The reactivity of the halogens DECREASES as you go down the group Covalent Compounds Ionic Compounds Learn the key facts about ionic compounds: • They are between non–metal elements – like hydrogen , oxygen and carbon • Electrons are SHARED between the electrons. • Carbon makes 4 bonds, nitrogen 3, oxygen 2, hydrogen and the halogens 1 • They are usually ‘simple’ molecules with a few atoms joined • They are gases as they have low boiling points • Examples include: O 2, CH 4, NH 3 • They are between a metal element and a non – metal element (eg: sodium chloride) • Electrons are TRANSFERRED. Metals always LOSE electrons. Non-Metals gain them • They are ‘salts’ • They are solid • They have high melting and boiling points • They cannot conduct electricity when they are solid • They can conduct electricity if you melt them or dissolve them in water • They are made of millions of atoms
- Slides: 2