Knowledge Organiser CHEMISTRY Unit 2 Structure and Bonding
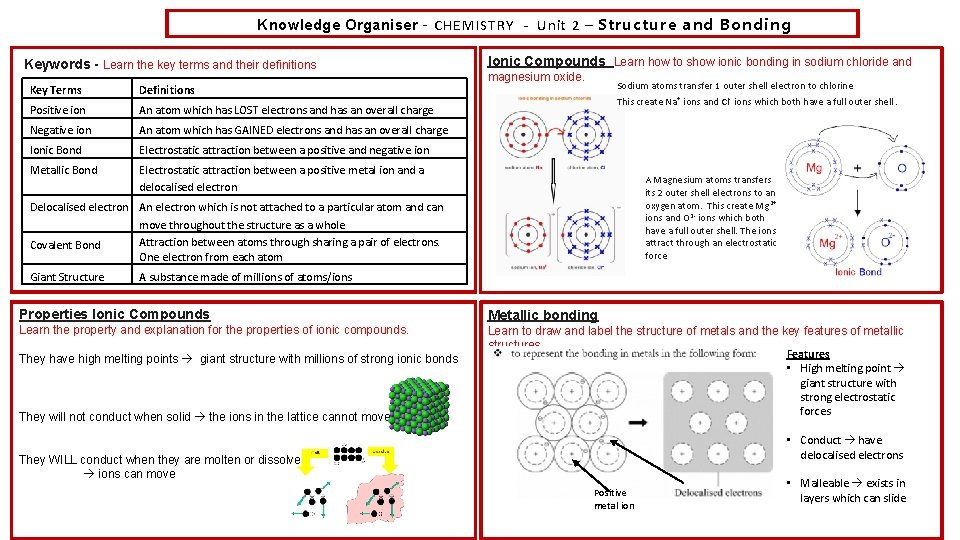
Knowledge Organiser – CHEMISTRY - Unit 2 – Structure and Bonding Keywords - Learn the key terms and their definitions Key Terms Definitions Positive ion An atom which has LOST electrons and has an overall charge Negative ion An atom which has GAINED electrons and has an overall charge Ionic Bond Electrostatic attraction between a positive and negative ion Metallic Bond Electrostatic attraction between a positive metal ion and a delocalised electron Ionic Compounds Learn how to show ionic bonding in sodium chloride and magnesium oxide. Sodium atoms transfer 1 outer shell electron to chlorine This create Na + ions and Cl - ions which both have a full outer shell. A Magnesium atoms transfers its 2 outer shell electrons to an oxygen atom. This create Mg 2+ ions and O 2 - ions which both have a full outer shell. The ions attract through an electrostatic force Delocalised electron An electron which is not attached to a particular atom and can move throughout the structure as a whole Attraction between atoms through sharing a pair of electrons. Covalent Bond One electron from each atom Giant Structure A substance made of millions of atoms/ions Properties Ionic Compounds Metallic bonding Learn the property and explanation for the properties of ionic compounds. Learn to draw and label the structure of metals and the key features of metallic structures. Features • High melting point giant structure with strong electrostatic forces They have high melting points giant structure with millions of strong ionic bonds They will not conduct when solid the ions in the lattice cannot move • Conduct have delocalised electrons They WILL conduct when they are molten or dissolved ions can move Positive metal ion • Malleable exists in layers which can slide
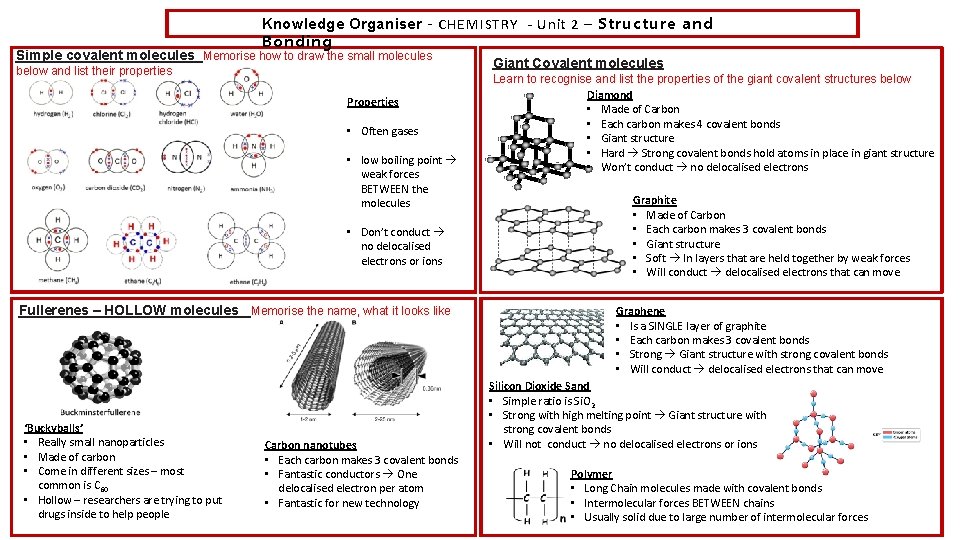
Knowledge Organiser – CHEMISTRY - Unit 2 – Structure and Bonding Simple covalent molecules Memorise how to draw the small molecules below and list their properties Properties • Often gases • low boiling point weak forces BETWEEN the molecules • Don’t conduct no delocalised electrons or ions Fullerenes – HOLLOW molecules Memorise the name, what it looks like ‘Buckyballs’ • Really small nanoparticles • Made of carbon • Come in different sizes – most common is C 60 • Hollow – researchers are trying to put drugs inside to help people Carbon nanotubes • Each carbon makes 3 covalent bonds • Fantastic conductors One delocalised electron per atom • Fantastic for new technology Giant Covalent molecules Learn to recognise and list the properties of the giant covalent structures below Diamond • Made of Carbon • Each carbon makes 4 covalent bonds • Giant structure • Hard Strong covalent bonds hold atoms in place in giant structure • Won’t conduct no delocalised electrons Graphite • Made of Carbon • Each carbon makes 3 covalent bonds • Giant structure • Soft In layers that are held together by weak forces • Will conduct delocalised electrons that can move Graphene • Is a SINGLE layer of graphite • Each carbon makes 3 covalent bonds • Strong Giant structure with strong covalent bonds • Will conduct delocalised electrons that can move Silicon Dioxide Sand • Simple ratio is Si. O 2 • Strong with high melting point Giant structure with strong covalent bonds • Will not conduct no delocalised electrons or ions Polymer • Long Chain molecules made with covalent bonds • Intermolecular forces BETWEEN chains • Usually solid due to large number of intermolecular forces
- Slides: 2