Knowledge Organiser CHEMISTRY Unit 1 Atomic Structure and
- Slides: 2

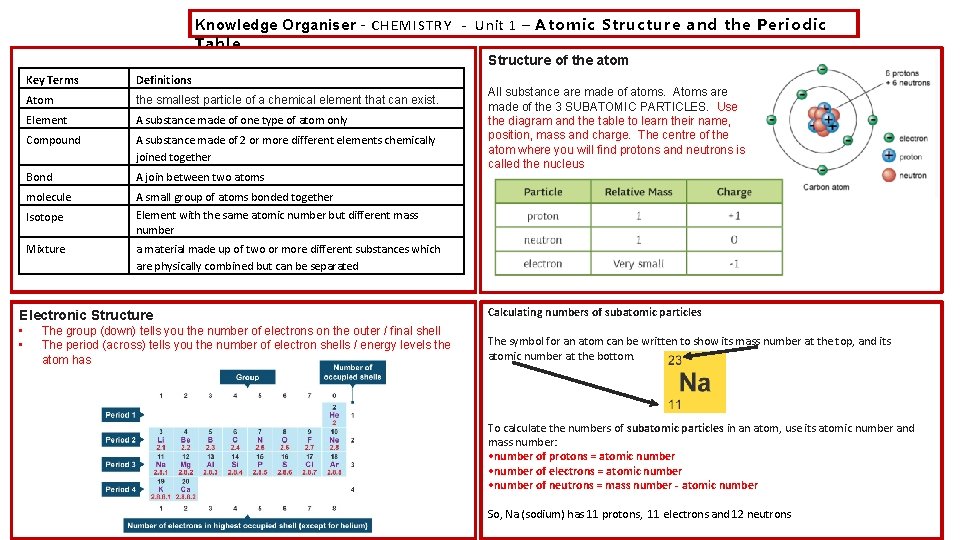
Knowledge Organiser – CHEMISTRY - Unit 1 – Atomic Structure and the Periodic Table Structure of the atom Key Terms Definitions Atom the smallest particle of a chemical element that can exist. Element A substance made of one type of atom only Compound A substance made of 2 or more different elements chemically joined together Bond A join between two atoms molecule A small group of atoms bonded together Element with the same atomic number but different mass number Isotope Mixture a material made up of two or more different substances which are physically combined but can be separated Electronic Structure • • All substance are made of atoms. Atoms are made of the 3 SUBATOMIC PARTICLES. Use the diagram and the table to learn their name, position, mass and charge. The centre of the atom where you will find protons and neutrons is called the nucleus The group (down) tells you the number of electrons on the outer / final shell The period (across) tells you the number of electron shells / energy levels the atom has Calculating numbers of subatomic particles The symbol for an atom can be written to show its mass number at the top, and its atomic number at the bottom. To calculate the numbers of subatomic particles in an atom, use its atomic number and mass number: • number of protons = atomic number • number of electrons = atomic number • number of neutrons = mass number - atomic number So, Na (sodium) has 11 protons, 11 electrons and 12 neutrons

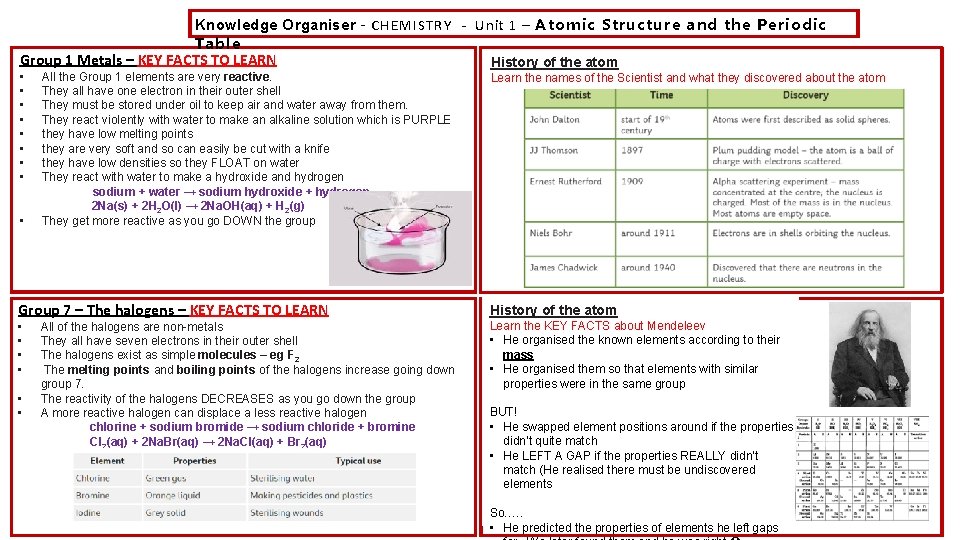
Knowledge Organiser – CHEMISTRY - Unit 1 – Atomic Structure and the Periodic Table Group 1 Metals – KEY FACTS TO LEARN History of the atom • • • All the Group 1 elements are very reactive. They all have one electron in their outer shell They must be stored under oil to keep air and water away from them. They react violently with water to make an alkaline solution which is PURPLE they have low melting points they are very soft and so can easily be cut with a knife they have low densities so they FLOAT on water They react with water to make a hydroxide and hydrogen sodium + water → sodium hydroxide + hydrogen 2 Na(s) + 2 H 2 O(l) → 2 Na. OH(aq) + H 2(g) They get more reactive as you go DOWN the group Learn the names of the Scientist and what they discovered about the atom Group 7 – The halogens – KEY FACTS TO LEARN History of the atom • • Learn the KEY FACTS about Mendeleev • He organised the known elements according to their mass • He organised them so that elements with similar properties were in the same group • • All of the halogens are non-metals They all have seven electrons in their outer shell The halogens exist as simple molecules – eg F 2 The melting points and boiling points of the halogens increase going down group 7. The reactivity of the halogens DECREASES as you go down the group A more reactive halogen can displace a less reactive halogen chlorine + sodium bromide → sodium chloride + bromine Cl 2(aq) + 2 Na. Br(aq) → 2 Na. Cl(aq) + Br 2(aq) BUT! • He swapped element positions around if the properties didn’t quite match • He LEFT A GAP if the properties REALLY didn’t match (He realised there must be undiscovered elements So…. . • He predicted the properties of elements he left gaps