Klinikum Rosenheim CR T 20 1 1 Department











- Slides: 23

Klinikum Rosenheim CR T 20 1 1 Department of Diagnostic and Interventional Radiology Current Results of DEB Systems in the SFA and Tibial Arteries Gunnar Tepe

Gunnar Tepe, MD § Grants/Contracted Research – Abbott Vascular – Medrad, Inc. – Invatec Technology Center, Gmb. H – Cook Medical – Lutonix, Inc. I intend to reference off label or unapproved uses of drugs or devices in my presentation. I intend to discuss Data from studies but with no FDA approval until now

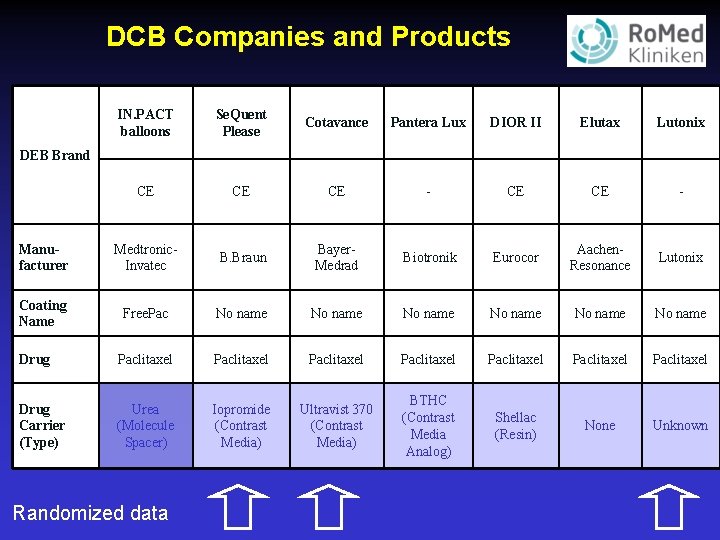
DCB Companies and Products IN. PACT balloons Se. Quent Please Cotavance Pantera Lux DIOR II Elutax Lutonix CE CE CE - Manufacturer Medtronic. Invatec B. Braun Bayer. Medrad Biotronik Eurocor Aachen. Resonance Lutonix Coating Name Free. Pac No name No name Drug Paclitaxel Paclitaxel Drug Carrier (Type) Urea (Molecule Spacer) Iopromide (Contrast Media) Ultravist 370 (Contrast Media) BTHC (Contrast Media Analog) Shellac (Resin) None Unknown DEB Brand Randomized data

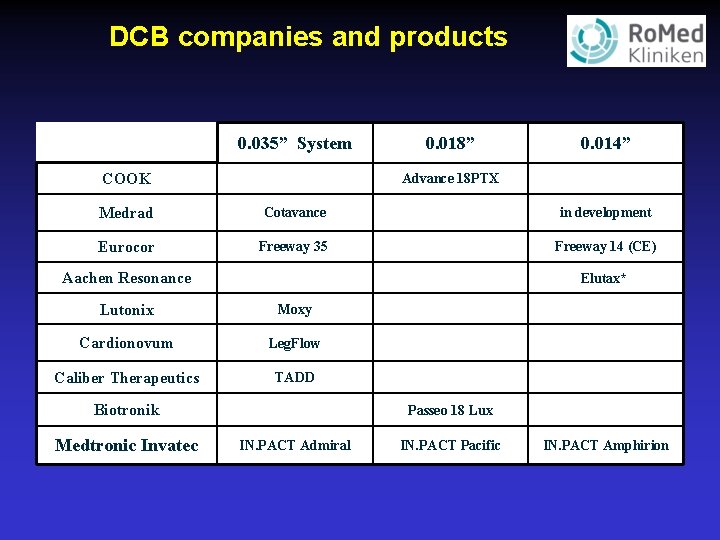
DCB companies and products 0. 035” System 0. 018” 0. 014” COOK Advance 18 PTX Medrad Cotavance in development Eurocor Freeway 35 Freeway 14 (CE) Aachen Resonance Elutax* Lutonix Moxy Cardionovum Leg. Flow Caliber Therapeutics TADD Biotronik Medtronic Invatec Passeo 18 Lux IN. PACT Admiral IN. PACT Pacific IN. PACT Amphirion

Study types 1. Proof that the DEB is safe and effective (DEB vs bare balloon) Paccocath – Cotavance technology (Thunder, Fem. Pac trial) Lutonix – Levant 1 2. New indications a) SFA - In stent restenosis b) SFA - With stents c) SFA - with atherectomy d) BTK e) AV-Fistula 3. For US approval (DEB vs bare balloon)

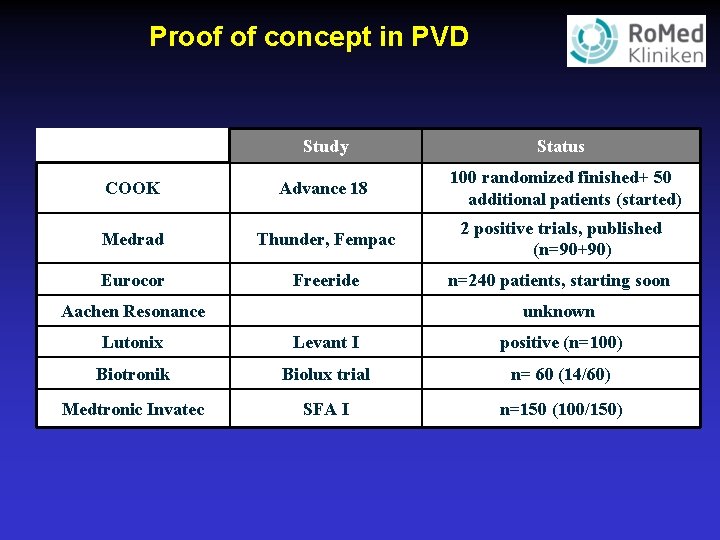
Proof of concept in PVD Study Status COOK Advance 18 100 randomized finished+ 50 additional patients (started) Medrad Thunder, Fempac 2 positive trials, published (n=90+90) Eurocor Freeride n=240 patients, starting soon Aachen Resonance unknown Lutonix Levant I positive (n=100) Biotronik Biolux trial n= 60 (14/60) Medtronic Invatec SFA I n=150 (100/150)

Randomized prospective SFA studies

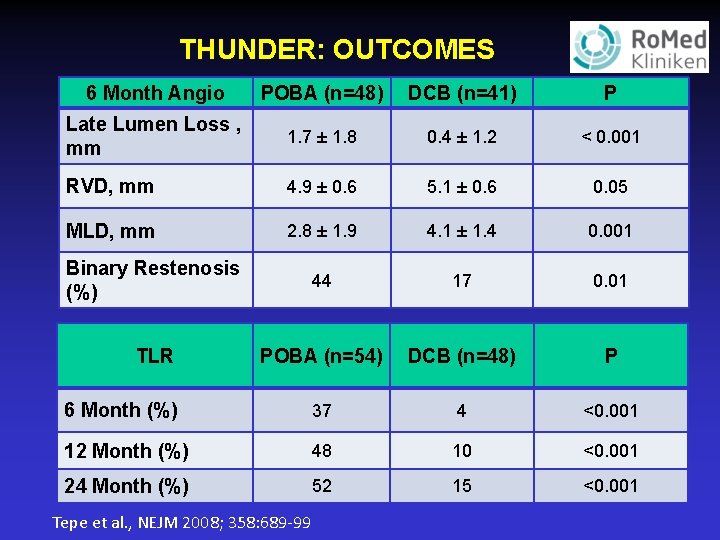
THUNDER: OUTCOMES 6 Month Angio POBA (n=48) DCB (n=41) P Late Lumen Loss , mm 1. 7 ± 1. 8 0. 4 ± 1. 2 < 0. 001 RVD, mm 4. 9 ± 0. 6 5. 1 ± 0. 6 0. 05 MLD, mm 2. 8 ± 1. 9 4. 1 ± 1. 4 0. 001 Binary Restenosis (%) 44 17 0. 01 TLR POBA (n=54) DCB (n=48) P 6 Month (%) 37 4 <0. 001 12 Month (%) 48 10 <0. 001 24 Month (%) 52 15 <0. 001 Tepe et al. , NEJM 2008; 358: 689 -99

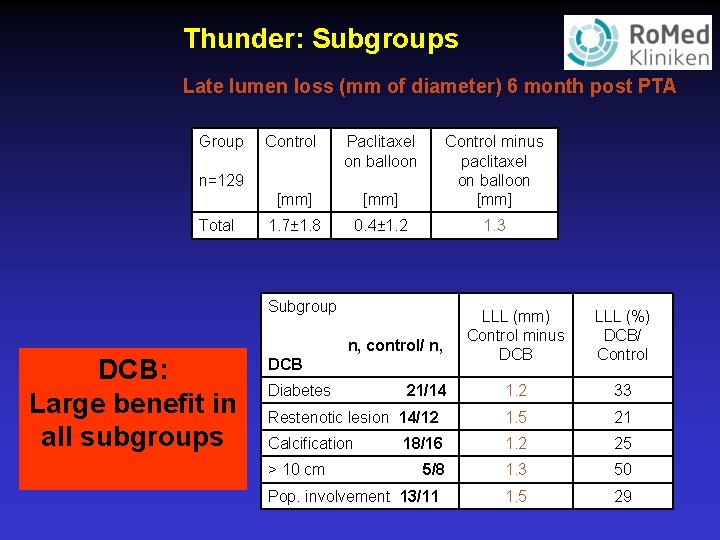
Thunder: Subgroups Late lumen loss (mm of diameter) 6 month post PTA Group Control Paclitaxel on balloon [mm] Control minus paclitaxel on balloon [mm] 1. 7± 1. 8 0. 4± 1. 2 1. 3 n=129 Total Subgroup DCB: Large benefit in all subgroups LLL (mm) Control minus DCB LLL (%) DCB/ Control 1. 2 33 Restenotic lesion 14/12 1. 5 21 Calcification 18/16 1. 2 25 5/8 1. 3 50 1. 5 29 n, control/ n, DCB Diabetes > 10 cm 21/14 Pop. involvement 13/11

Fem. Pac: OUTCOMES 6 Month Angio POBA (n=48) DCB (n=41) P Late Lumen Loss , mm 0. 8 0. 3 0. 031 RVD, mm 5. 1 5. 2 0. 62 MLD, mm 2. 7 3. 6 0. 037 Binary Restenosis (%) 47 19 0. 035 TLR POBA (n=54) DCB (n=48) P 6 Months (%) 33 7 0. 002 18 -24 Months (%) 50 13 0. 001 Werk et al. , CIRC 2008; 118: 1358 -1365

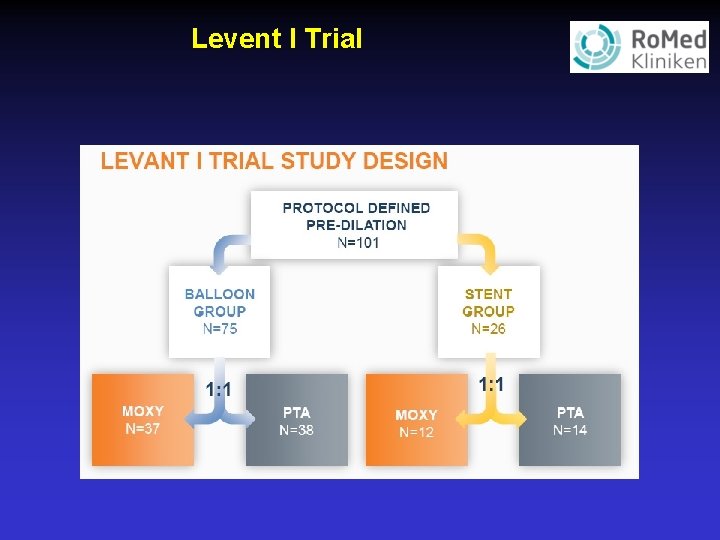
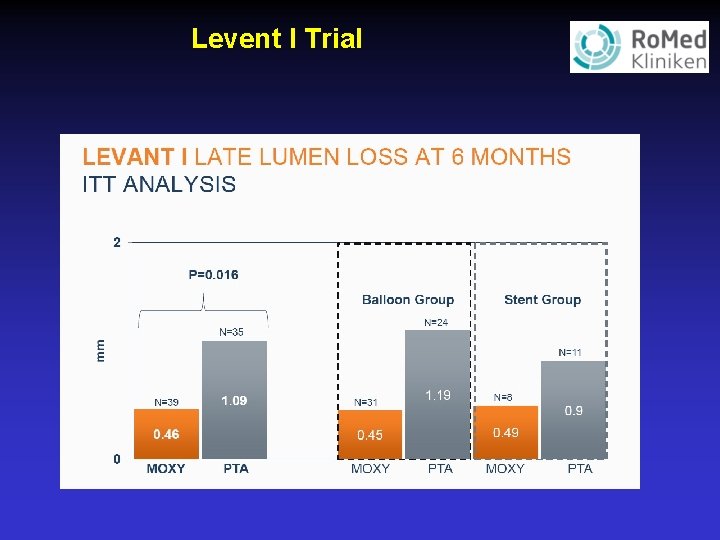
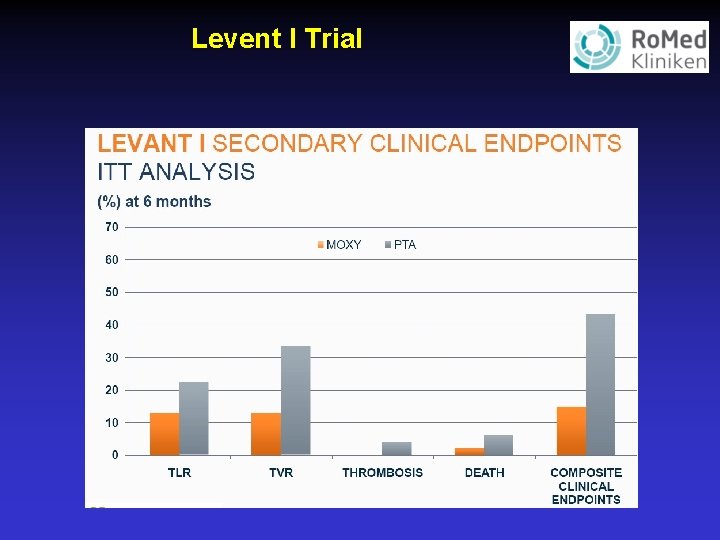
Levent I Trial

Levent I Trial

Levent I Trial

Lesions Learnt DEB: - Many DEBs available, but different - Paccocath and Lutonix with positive data - Several trials under the way

Non-randomized results

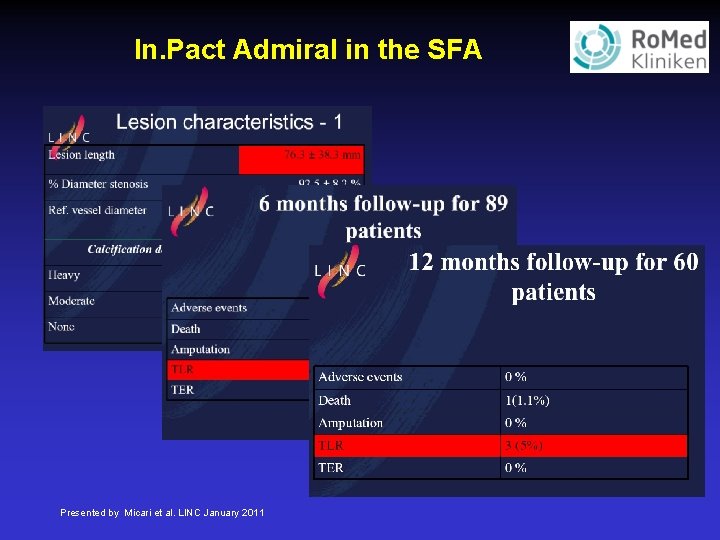
In. Pact Admiral in the SFA Presented by Micari et al. LINC January 2011

In. Pact Admiral in the SFA Presented by Micari et al. LINC January 2011

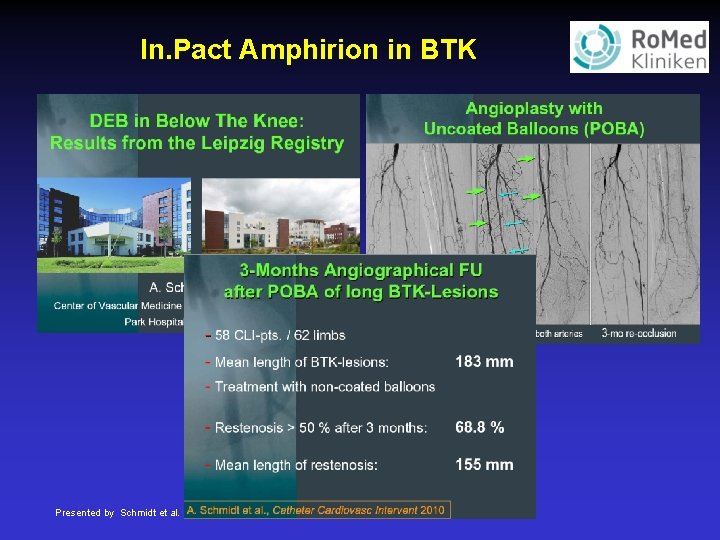
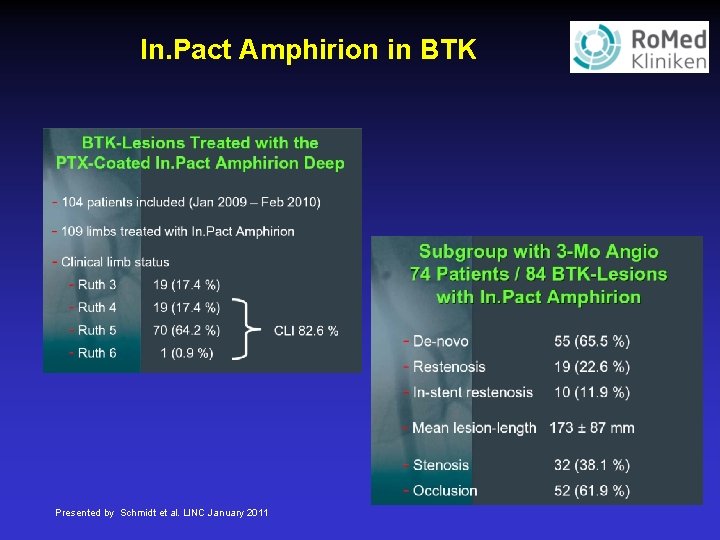
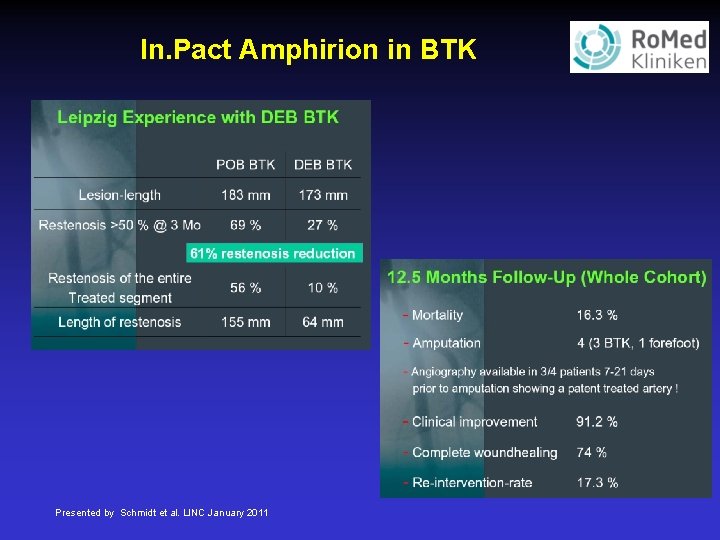
In. Pact Amphirion in BTK Presented by Schmidt et al. LINC January 2011

In. Pact Amphirion in BTK Presented by Schmidt et al. LINC January 2011

In. Pact Amphirion in BTK Presented by Schmidt et al. LINC January 2011

Further indications COOK Medrad Indication Name - - In-stent RS SFA BTK COPA COBANA (0/70) 6 mo DSA Euro Canal US Canal Definitve AR (0/100) 12 mo LLL Atherectomy (+EV 3) ? In-stent RS BTK? Freeway (ca. x/260) in discussion Aachen Resonance ? In-stent RS? - Lutonix - - Biotronik - - Medtronic Invatec In-stent RS SFA BTK Shunt FAIR (23/118) 6 mo DUS Eurocor + Stent In Pact Deep (140/357) 12 Mo DSA + clinical Inpact Shunt (0/136) 6 mo LLL

Race to the US market Not easy to be quick: FDA reqirements

Race to the US market Use the Thunder and Fem. Pac Data Indepented US trial planned (River Trial) Levant II is planned based on Levant I SFA II is planned using SFA I
 Klinikum lüdenscheid intensivstation
Klinikum lüdenscheid intensivstation Ameos hildesheim aufnahme
Ameos hildesheim aufnahme Organigramm bistum fulda
Organigramm bistum fulda Btk rosenheim
Btk rosenheim Amt für ernährung landwirtschaft und forsten rosenheim
Amt für ernährung landwirtschaft und forsten rosenheim Kindernephrologie münchen
Kindernephrologie münchen Stadtbibliothek rosenheim katalog
Stadtbibliothek rosenheim katalog Btk rosenheim
Btk rosenheim Amt für ernährung landwirtschaft und forsten rosenheim
Amt für ernährung landwirtschaft und forsten rosenheim Mittelschule am luitpoldpark rosenheim
Mittelschule am luitpoldpark rosenheim Schulamt rosenheim
Schulamt rosenheim Ift rosenheim
Ift rosenheim Marketing workshop agenda
Marketing workshop agenda Haverford biology
Haverford biology Bmc legal department
Bmc legal department Oklahoma department of career and technology education
Oklahoma department of career and technology education California department of general services
California department of general services Urgent care centre whittington
Urgent care centre whittington University of sargodha engineering department
University of sargodha engineering department Yolo county planning department
Yolo county planning department Weights and measures california
Weights and measures california Job description for grouping tasks into departments
Job description for grouping tasks into departments Fresno county probation office
Fresno county probation office Nys dept of homeland security
Nys dept of homeland security