Kinetics Rates of Chemical Reactions Dr Ron Rusay

















- Slides: 49

Kinetics Rates of Chemical Reactions Dr. Ron Rusay

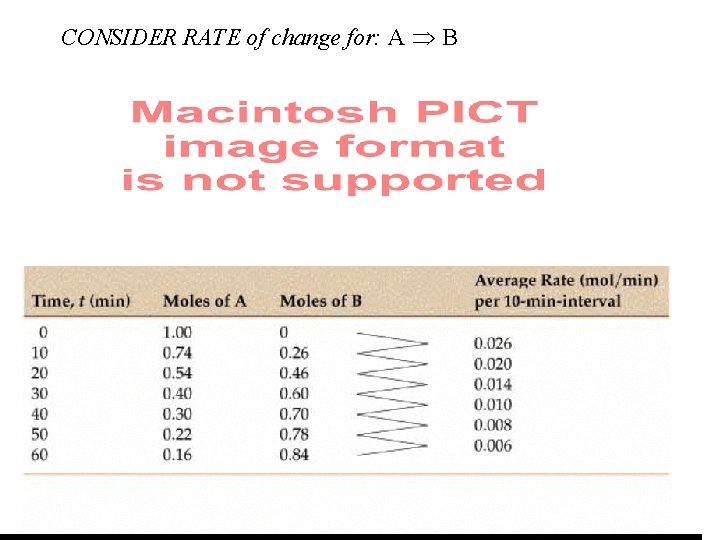
Chemical Reactions: Time & Progress A B Red Blue t (min) 100 molecules 54 Red : 46 Blue 30 Red : 70 Blue

Moles A & B vs. Time A B

QUESTION If we have the reaction A(g) 2 B(g) and the number of moles of A follows: time moles A 0. 100 0 0. 085 5 min 0. 070 10 min What is the number of moles of B at 10 min? A. B. C. D. E. 0. 060 mol 0. 200 mol 0. 140 mol 0. 100 mol 0. 030 mol

ANSWER If we have the reaction A(g) 2 B(g) and the number of moles of A is as follows, time moles A 0 0. 100 5 min 0. 085 10 min 0. 070 What is the number of moles of B at 10 min? A. B. C. D. E. 0. 060 mol 0. 200 mol 0. 140 mol 0. 100 mol 0. 030 mol 0. 100 mol. A -0. 070 mol. A 0. 030 mol. A x 2 mol. B/1 mol. A

CONSIDER RATE of change for: A B

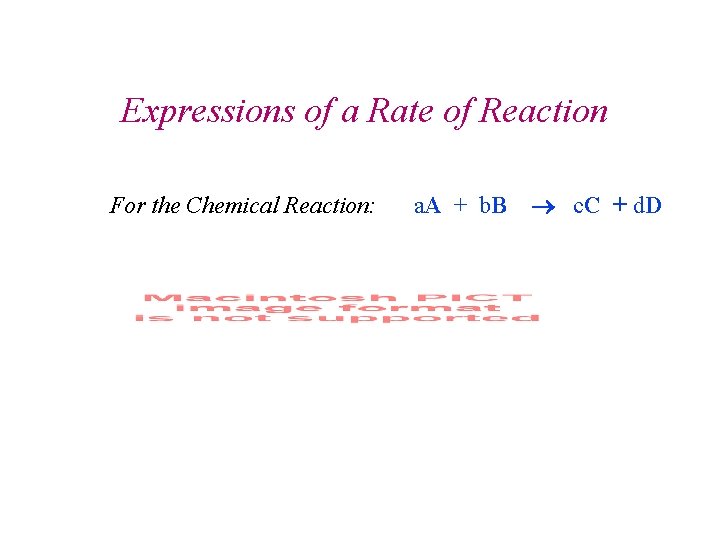
Expressions of a Rate of Reaction For the Chemical Reaction: a. A + b. B c. C + d. D

QUESTION Chlorine dioxide (Cl. O 2) is a disinfectant used in municipal watertreatment plants. It dissolves in basic solution producing Cl. O 3– and Cl. O 2–: 2 Cl. O 2 (aq) + 2 OH– (aq) Cl. O 3– (aq) + Cl. O 2– (aq) + H 2 O (l) Of the following, which would not be a proper expression to relate information about the rate of the reaction? • –DCl. O 2/Dt = 2 DCl. O 3–/Dt • –DCl. O 2/Dt = DOH–/Dt • –DCl. O 2/Dt = DCl. O 2–/Dt • –DOH–/Dt = 2 DCl. O 2–/Dt

ANSWER C. The stoichiometry of the reaction indicates that Cl. O 2 disappears twice as fast as Cl. O 2– forms; so to determine the rate for Cl. O 2 , the rate of Cl. O 2– would have to be doubled or ½ of the rate of disappearance of Cl. O 2.

Rate of Reaction C 4 H 9 Cl(aq) + H 2 O(l) C 4 H 9 OH(aq) + HCl(aq) � The average rate can be expressed in terms of the disappearance of C 4 H 9 Cl. � The units for average rate are mol/L. s or M/s. � The average rate decreases over time.

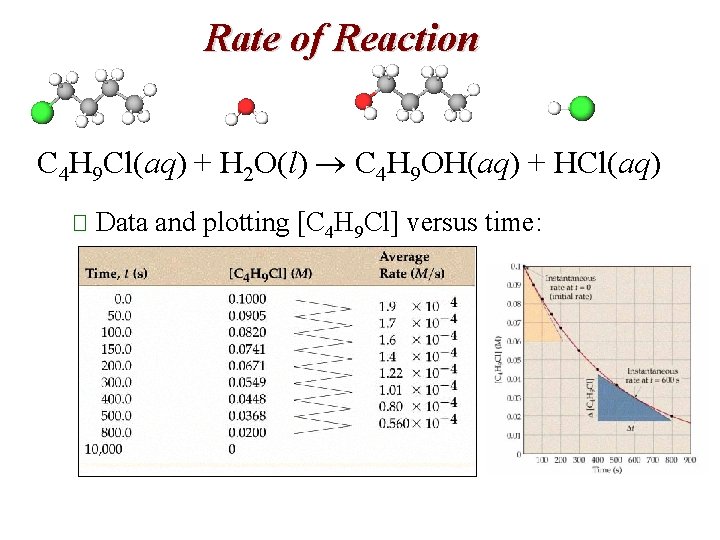
Rate of Reaction C 4 H 9 Cl(aq) + H 2 O(l) C 4 H 9 OH(aq) + HCl(aq) � Data and plotting [C 4 H 9 Cl] versus time:

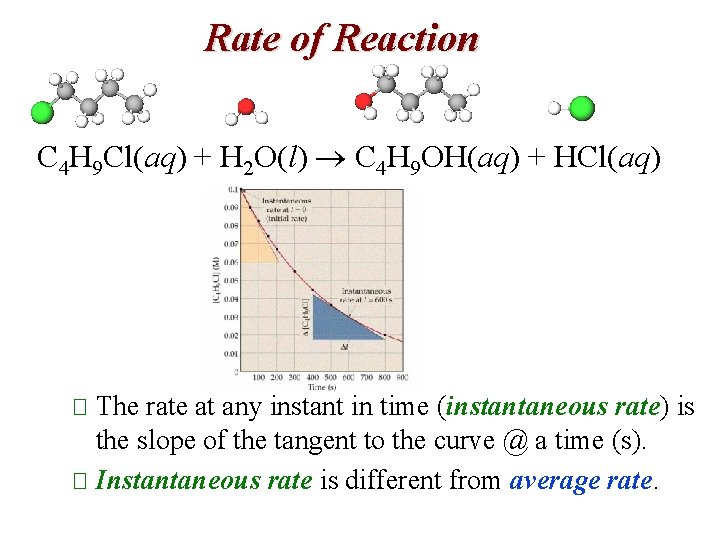
Rate of Reaction C 4 H 9 Cl(aq) + H 2 O(l) C 4 H 9 OH(aq) + HCl(aq) � The rate at any instant in time (instantaneous rate) is the slope of the tangent to the curve @ a time (s). � Instantaneous rate is different from average rate.

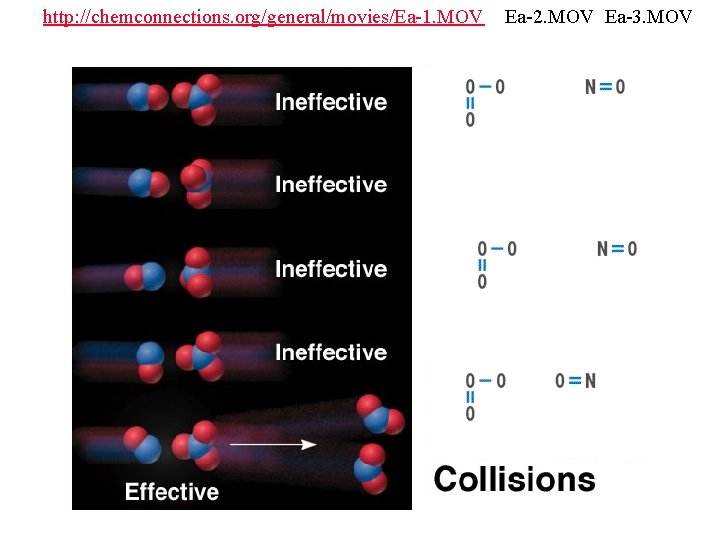
http: //chemconnections. org/general/movies/Ea-1. MOV Ea-2. MOV Ea-3. MOV

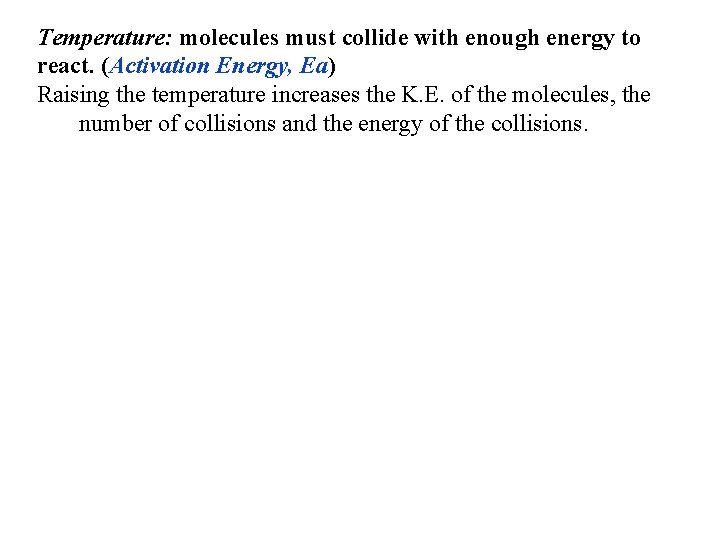
Indicator Kinetics Crystal Violet + OH Phenolphthalein + H 2 O - Color Changes & Concentrations

Important Factors that Effect Reaction Rates 1) Concentration: molecules must collide in order to react. The higher the concentration, the higher number of collisions. Rate = k (collision frequency) = k (concentration) k = rate constant 2) Physical state: molecules must physically mix in order to collide. The physical state (solid, liquid, gas) will affect frequency of collisions, as well as the physical size of droplets (liquid) or particles in the case of solids. (heterogeneous vs. homogeneous) 3) Temperature: molecules must collide with enough energy to react. (Activation Energy, Ea) Raising the temperature increases the K. E. of the molecules, the number of collisions and the energy of the collisions.


Temperature: molecules must collide with enough energy to react. (Activation Energy, Ea) Raising the temperature increases the K. E. of the molecules, the number of collisions and the energy of the collisions.

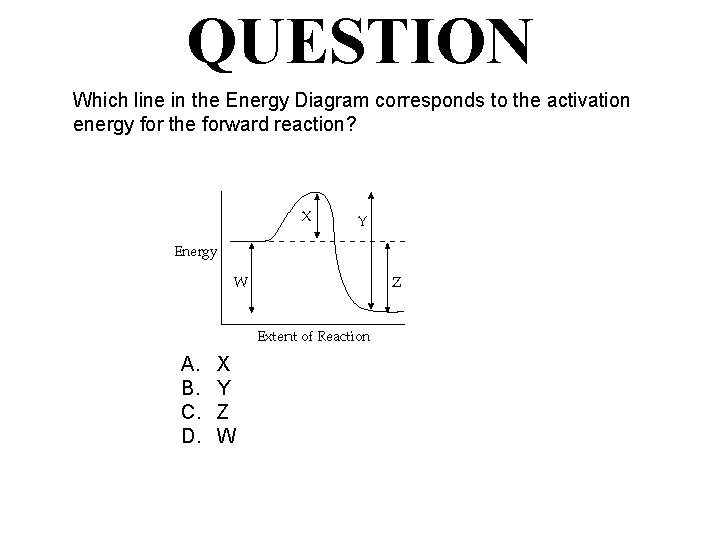
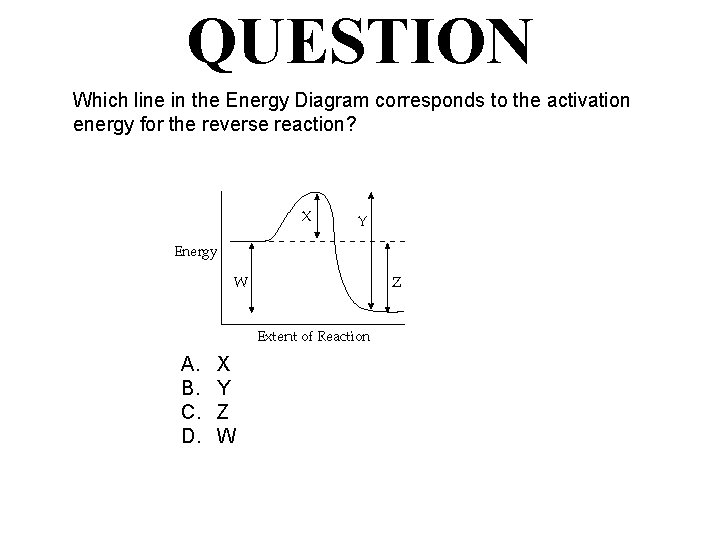
QUESTION Which line in the Energy Diagram corresponds to the activation energy for the forward reaction? A. B. C. D. X Y Z W

ANSWER Which line in the Energy Diagram corresponds to the activation energy for the reverse reaction? A. B. C. D. X Y Z W

QUESTION Which line in the Energy Diagram corresponds to the activation energy for the reverse reaction? A. B. C. D. X Y Z W

ANSWER Which line in the Energy Diagram corresponds to the activation energy for the reverse reaction? A. B. C. D. X Y Z W

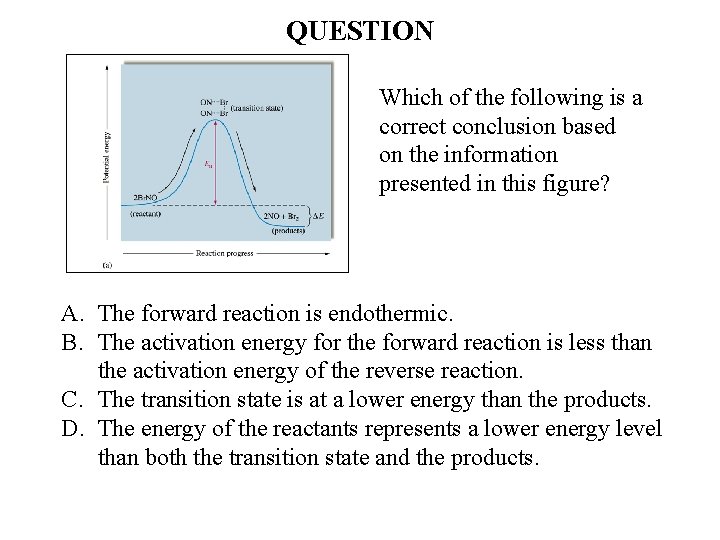
QUESTION Which of the following is a correct conclusion based on the information presented in this figure? A. The forward reaction is endothermic. B. The activation energy for the forward reaction is less than the activation energy of the reverse reaction. C. The transition state is at a lower energy than the products. D. The energy of the reactants represents a lower energy level than both the transition state and the products.

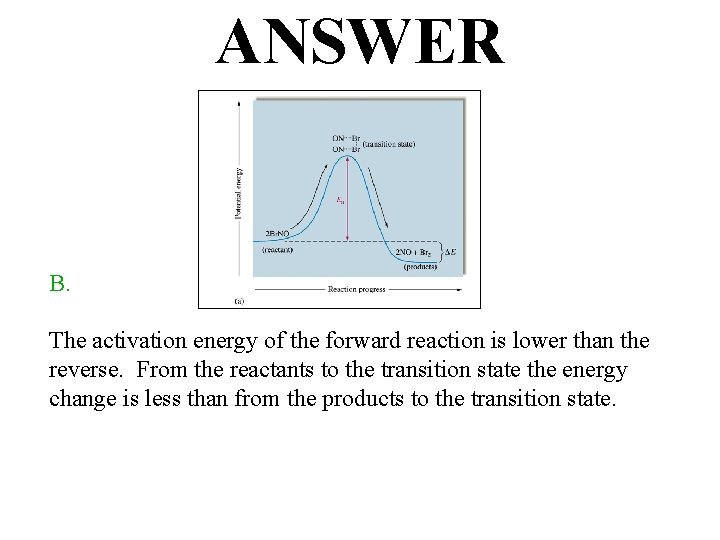
ANSWER B. The activation energy of the forward reaction is lower than the reverse. From the reactants to the transition state the energy change is less than from the products to the transition state.

Ph. ET Simulation https: //phet. colorado. edu/en/simulation/legacy/reactions-and-rates

Catalysts Alternative Reaction Pathways • Catalysts increase reaction rates by lowering the Energy of Activation Ea vs Ea • Catalysts are not consumed in chemical reactions

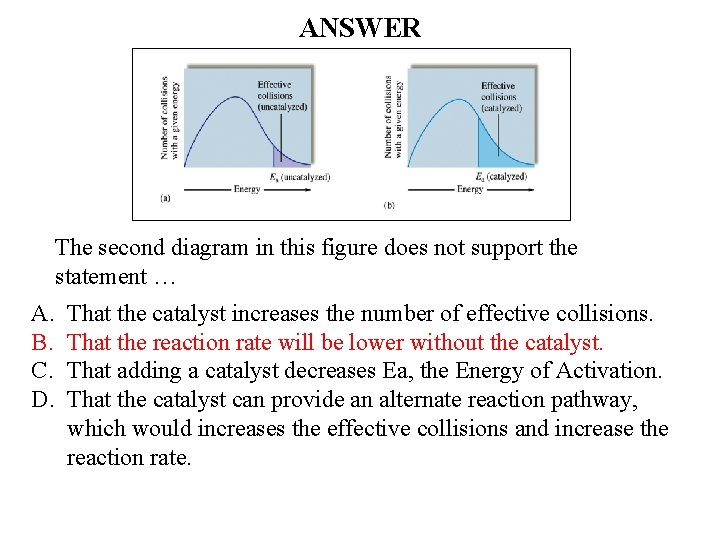
QUESTION The second diagram in this figure does not support the statement … A. B. C. D. That the catalyst increases the number of effective collisions. That the reaction rate will be lower without the catalyst. That adding a catalyst decreases Ea, the Energy of Activation. That the catalyst can provide an alternate reaction pathway, which would increases the effective collisions and increase the reaction rate.

ANSWER The second diagram in this figure does not support the statement … A. B. C. D. That the catalyst increases the number of effective collisions. That the reaction rate will be lower without the catalyst. That adding a catalyst decreases Ea, the Energy of Activation. That the catalyst can provide an alternate reaction pathway, which would increases the effective collisions and increase the reaction rate.

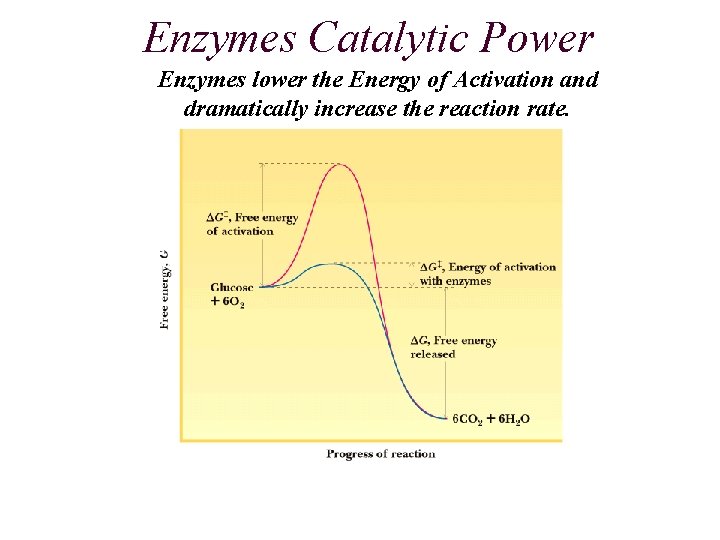
Enzymes Catalytic Power Enzymes lower the Energy of Activation and dramatically increase the reaction rate.

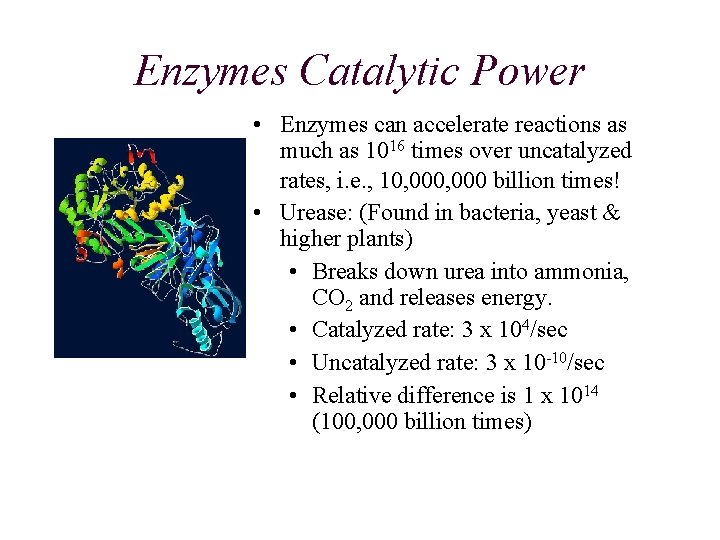
Enzymes Catalytic Power • Enzymes can accelerate reactions as much as 1016 times over uncatalyzed rates, i. e. , 10, 000 billion times! • Urease: (Found in bacteria, yeast & higher plants) • Breaks down urea into ammonia, CO 2 and releases energy. • Catalyzed rate: 3 x 104/sec • Uncatalyzed rate: 3 x 10 -10/sec • Relative difference is 1 x 1014 (100, 000 billion times)

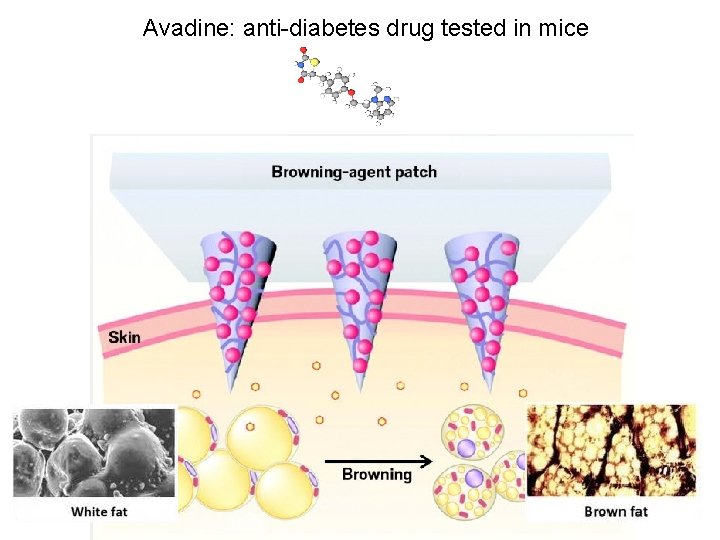
Avadine: anti-diabetes drug tested in mice

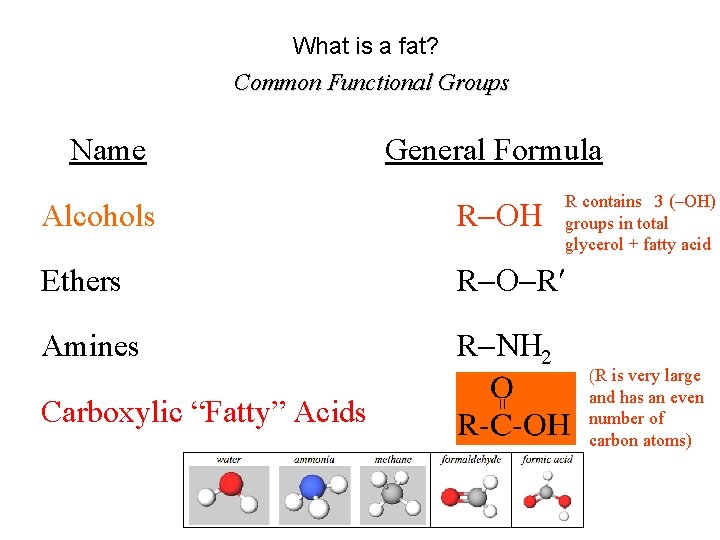
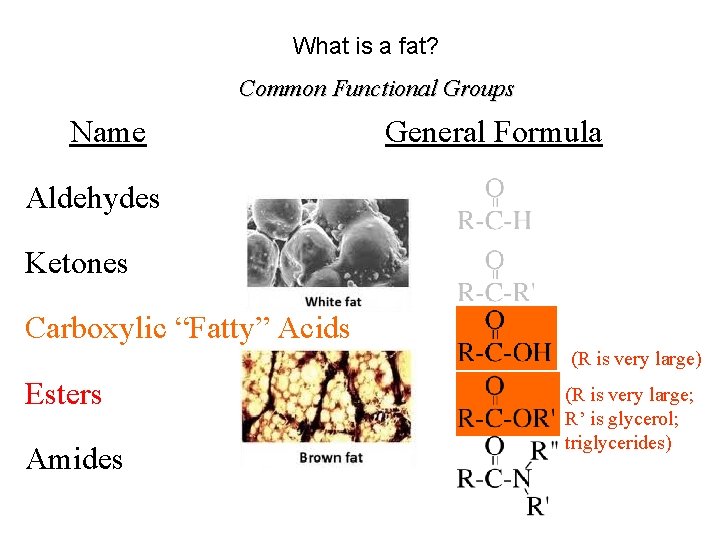
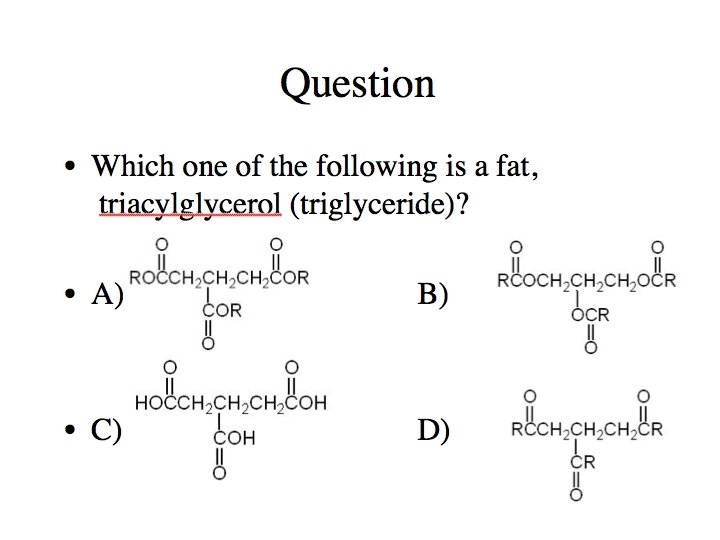
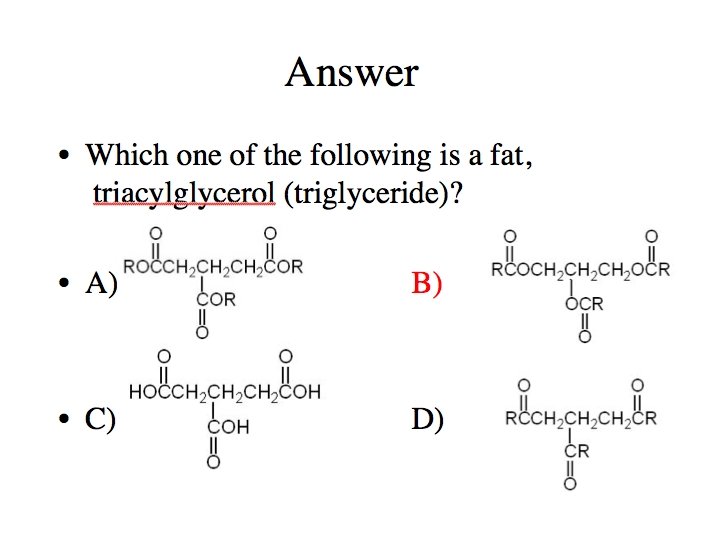
What is a fat? Common Functional Groups Name General Formula R contains 3 (–OH) groups in total glycerol + fatty acid Alcohols R Ethers R R Amines R N 2 Carboxylic “Fatty” Acids (R is very large and has an even number of carbon atoms)

What is a fat? Common Functional Groups Name General Formula Aldehydes Ketones Carboxylic “Fatty” Acids Esters Amides (R is very large) (R is very large; R’ is glycerol; triglycerides)

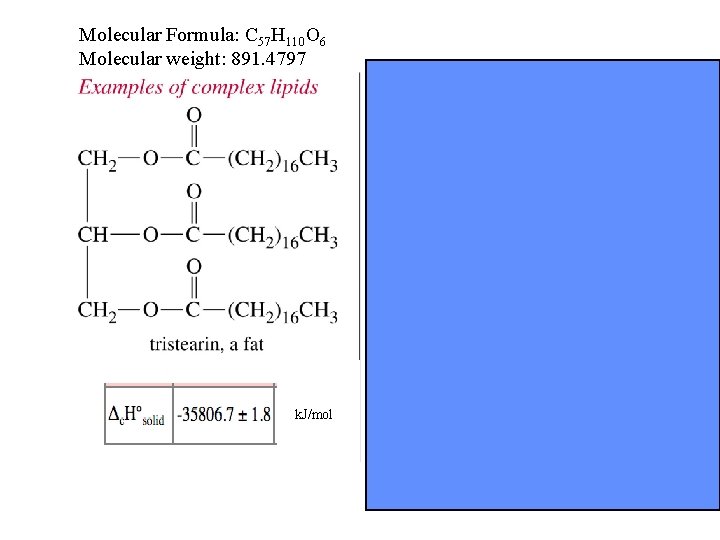
Molecular Formula: C 57 H 110 O 6 Molecular weight: 891. 4797 k. J/mol

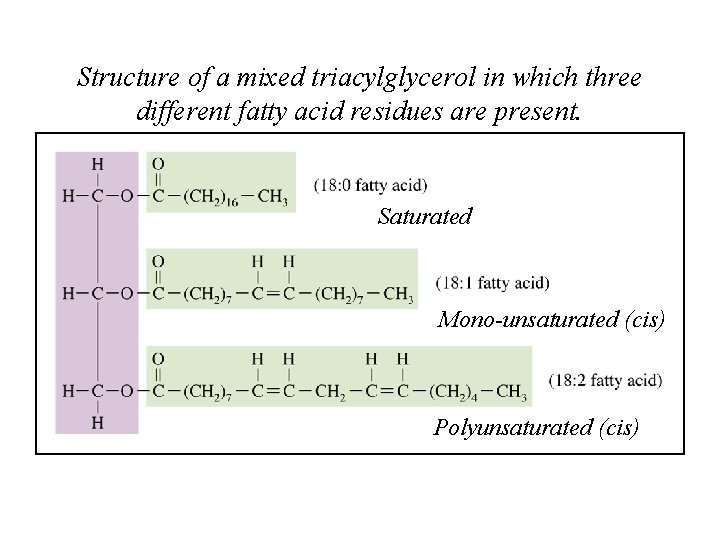
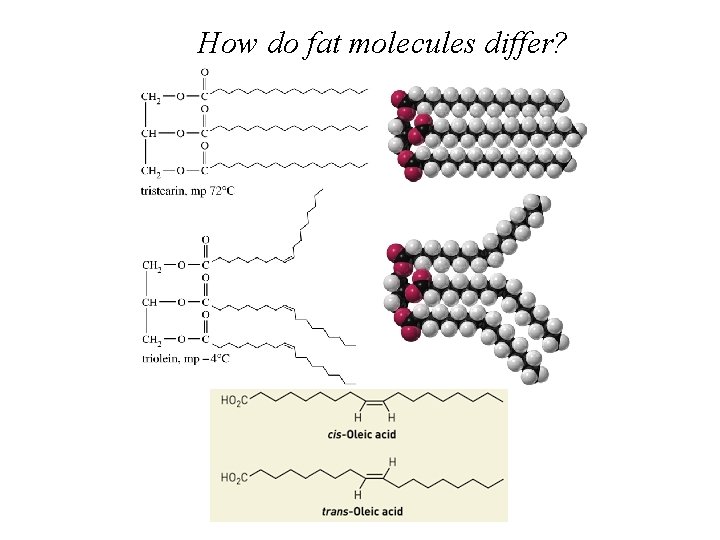
Structure of a mixed triacylglycerol in which three different fatty acid residues are present. Saturated Mono-unsaturated (cis) Polyunsaturated (cis)

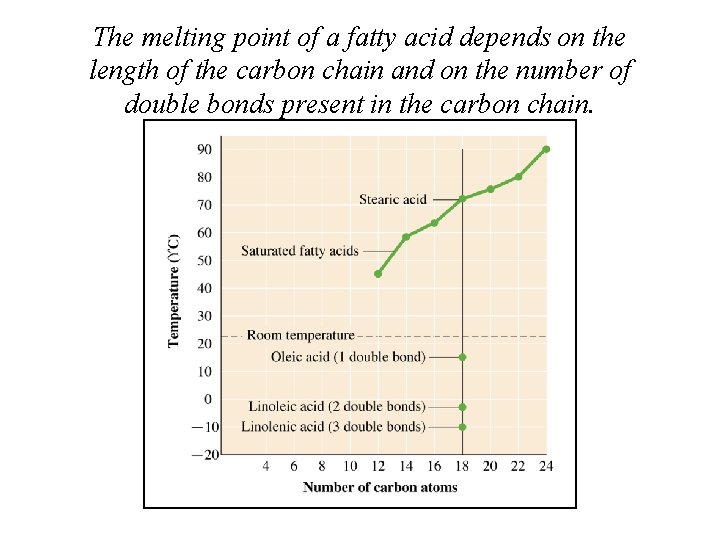
The melting point of a fatty acid depends on the length of the carbon chain and on the number of double bonds present in the carbon chain.

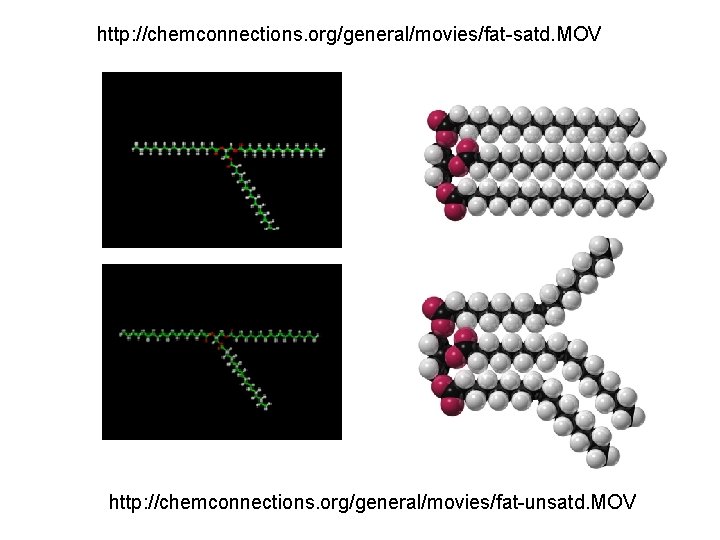
How do fat molecules differ?

http: //chemconnections. org/general/movies/fat-satd. MOV http: //chemconnections. org/general/movies/fat-unsatd. MOV

http: //chemconnections. org//general/chem 106/The%20 Worst%20 Fat%20 in%20 the%20 Food%20 Suppl y%20 -%20 The%20 New%20 York%20 Times. pdf

Question Which of the following statements regarding fatty acids is false? A) Fatty acid can have one or more carbon-carbon double bonds. B) Naturally occurring fatty acids have an odd number of carbons. C) The configuration of the double bond(s) is (are) generally cis in naturally occurring fatty acids. D) Unsaturated fatty acids have a lower melting point than saturated ones.

Answer Which of the following statements regarding fatty acids is false? A) Fatty acid can have one or more carbon-carbon double bonds. B) Naturally occurring fatty acids have an odd number of carbons. C) The configuration of the double bond(s) is (are) generally cis in naturally occurring fatty acids. D) Unsaturated fatty acids have a lower melting point than saturated ones.



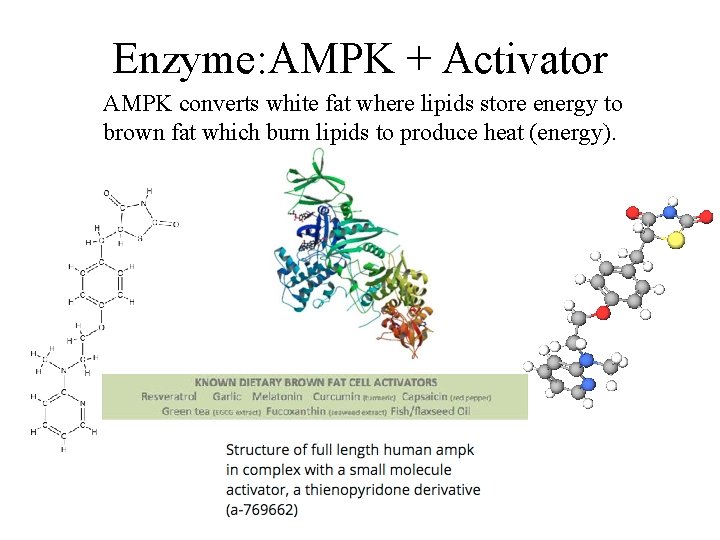
Enzyme: AMPK + Activator AMPK converts white fat where lipids store energy to brown fat which burn lipids to produce heat (energy).

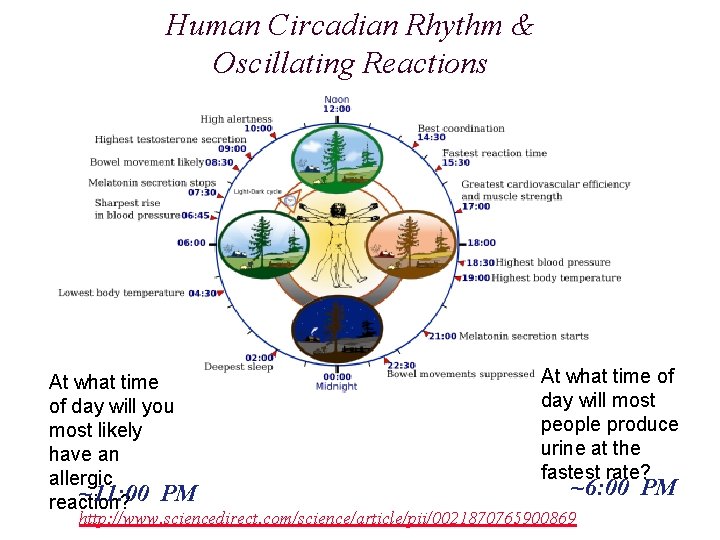
Human Circadian Rhythm & Oscillating Reactions At what time of day will you most likely have an allergic ~11: 00 PM reaction? At what time of day will most people produce urine at the fastest rate? ~6: 00 PM http: //www. sciencedirect. com/science/article/pii/0021870765900869

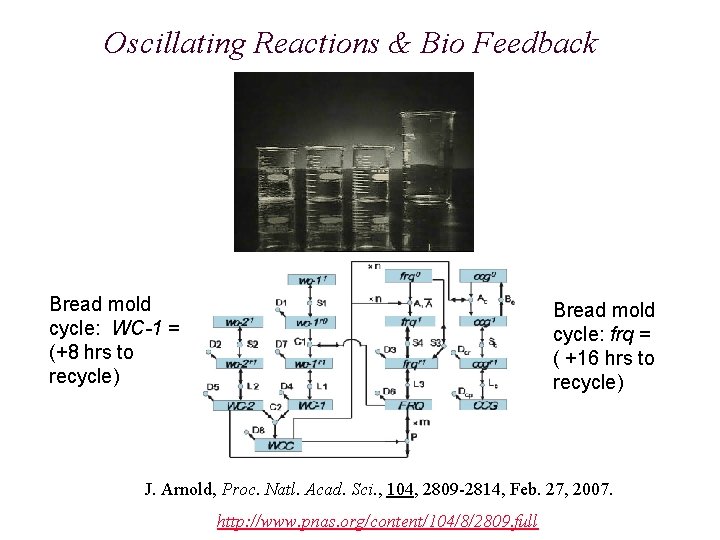
Oscillating Reactions & Bio Feedback Bread mold cycle: WC-1 = (+8 hrs to recycle) Bread mold cycle: frq = ( +16 hrs to recycle) J. Arnold, Proc. Natl. Acad. Sci. , 104, 2809 -2814, Feb. 27, 2007. http: //www. pnas. org/content/104/8/2809. full

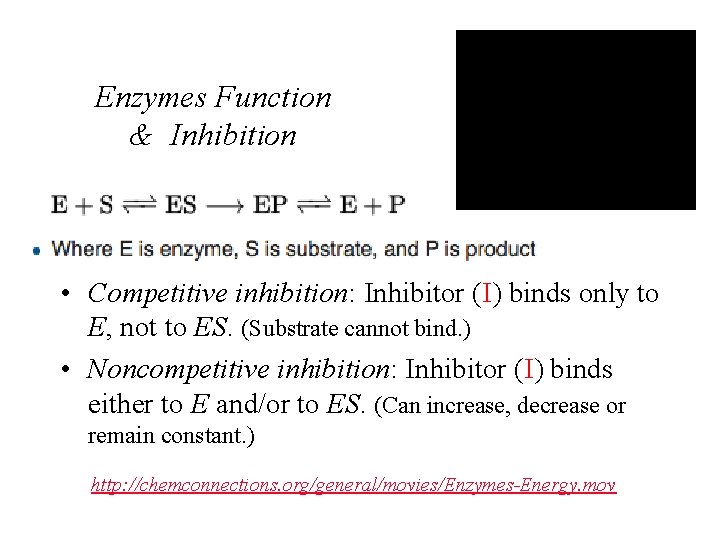
Enzymes Function & Inhibition • Competitive inhibition: Inhibitor (I) binds only to E, not to ES. (Substrate cannot bind. ) • Noncompetitive inhibition: Inhibitor (I) binds either to E and/or to ES. (Can increase, decrease or remain constant. ) http: //chemconnections. org/general/movies/Enzymes-Energy. mov

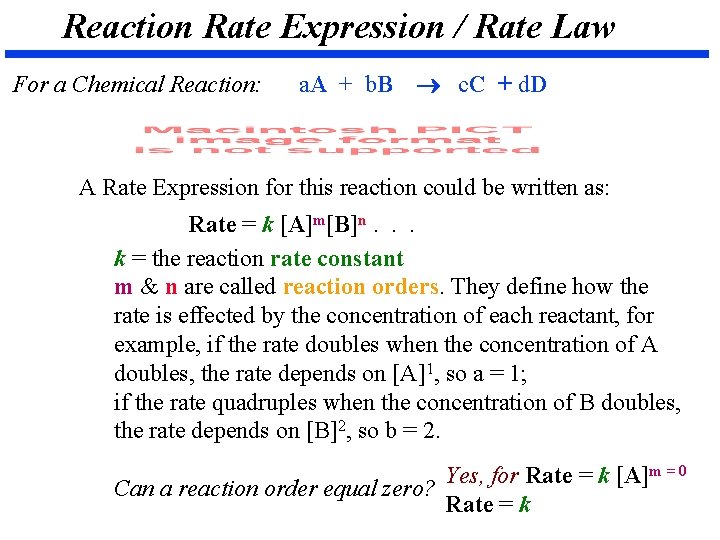
Reaction Rate Expression / Rate Law For a Chemical Reaction: a. A + b. B c. C + d. D A Rate Expression for this reaction could be written as: Rate = k [A]m[B]n. . . k = the reaction rate constant m & n are called reaction orders. They define how the rate is effected by the concentration of each reactant, for example, if the rate doubles when the concentration of A doubles, the rate depends on [A]1, so a = 1; if the rate quadruples when the concentration of B doubles, the rate depends on [B]2, so b = 2. Yes, for Rate = k [A]m = 0 Can a reaction order equal zero? Rate = k

Chemical Biology Reactions/Catalysts Globular Proteins / Enzymes Metabolism

Human Metabolism Defined by enzymes: globular proteins that catalyze all reactions & processes in human chemical biology
 Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Rusay
Rusay Types of reactions
Types of reactions Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Ratios guided notes
Ratios guided notes Ratios rates and unit rates
Ratios rates and unit rates Ratios rates and unit rates
Ratios rates and unit rates Unit rate vocabulary
Unit rate vocabulary Applications of chemical kinetics
Applications of chemical kinetics Chemical kinetics experiment
Chemical kinetics experiment Chemical reactions grade 11
Chemical reactions grade 11 Steady state approximation in chemical kinetics
Steady state approximation in chemical kinetics Half life chemical kinetics
Half life chemical kinetics Chemical kinetics definition
Chemical kinetics definition Molecularity of reaction
Molecularity of reaction Redox reactions examples
Redox reactions examples Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Toxic reactions chemical equations
Toxic reactions chemical equations Balancing equations chapter 8
Balancing equations chapter 8 5 general types of chemical reactions
5 general types of chemical reactions Equilibrium of chemical reactions
Equilibrium of chemical reactions What is this image
What is this image Four types of chemical reactions
Four types of chemical reactions Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry A balanced chemical reaction obeys the law of
A balanced chemical reaction obeys the law of Describing chemical reactions
Describing chemical reactions 4 types of chemical reactions
4 types of chemical reactions Chapter 11 chemical reactions answer key
Chapter 11 chemical reactions answer key Summary of chemical reactions
Summary of chemical reactions Chemical reactions that release energy are called
Chemical reactions that release energy are called Stoichiometry island diagram
Stoichiometry island diagram Four types of chemical reactions
Four types of chemical reactions Solvent in chemical reactions
Solvent in chemical reactions Solvent in chemical reactions
Solvent in chemical reactions Understanding chemical reactions worksheet answer key
Understanding chemical reactions worksheet answer key Examples of chemical reactions in everyday life
Examples of chemical reactions in everyday life Combination reaction equation
Combination reaction equation Chapter 19 chemical reactions answer key
Chapter 19 chemical reactions answer key Three types of chemical reactions
Three types of chemical reactions Enzymes affect the reactions in living cells by
Enzymes affect the reactions in living cells by 4 types of chemical reactions
4 types of chemical reactions Chapter 9 chemical reactions
Chapter 9 chemical reactions Chemical equations worksheet
Chemical equations worksheet 5 chemical reactions
5 chemical reactions Solubility rules
Solubility rules Chapter 9 chemical reactions
Chapter 9 chemical reactions What are protonic and non protonic solvents
What are protonic and non protonic solvents What is the role of enzymes in chemical reactions
What is the role of enzymes in chemical reactions