Kinetics of fast reactions Dr Teena Liz Luke













- Slides: 18

Kinetics of fast reactions Dr. Teena Liz Luke Assistant Professor in Chemistry Christian College Chengannur

Kinetics of fast reactions Kinetic methods are the measurement of the analytical signal is made under dynamic conditions in which the concentrations of reactants and products are changing as a function of time. Fast reactions are the chemical reactions which take place at a very fast rate. These reactions can take place in seconds or in minutes. In general the reactions between ionic compounds are fast.

Methods used for find the rate of fast reactions • • • Flow method Relaxation method Shock Method Flash Photolysis Pulser Laser method etc.

1. Flow Method • Continuous Flow Method • Stopped Flow Method

Continuous Flow Method • This technique is employed for both gas and liquid phase • Eg. Reaction between NO and O 2

• The two solutions are placed in separate containers and driven through aspecial mixing chamber into an observation tube • Composition of the solution is determined by optical, thermal or other methods • At various distances a concentration-time curve can be determined. • The measurements can made at a fixed distance and the velocity of flow varied

Apparatus for Continuous Flow Method

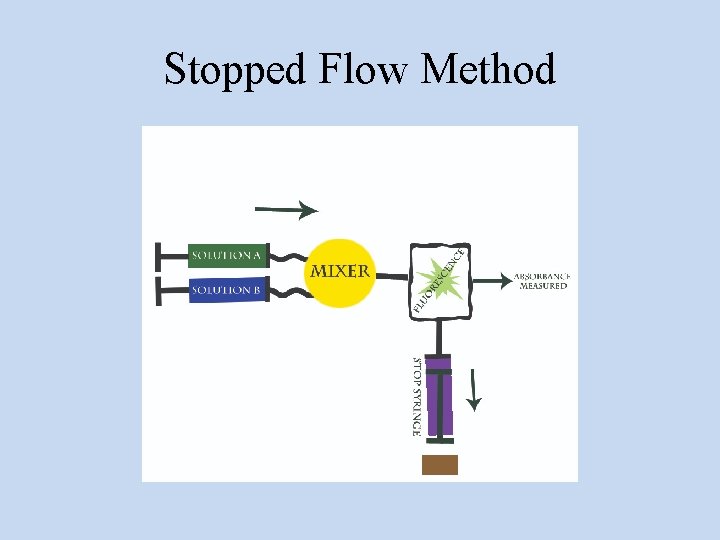
Stopped Flow Method

• The flow is stopped suddenly and the measurements are made spectrophotometrically of concentration as a function of time. • Since the reactions are rapid the spectrophotometric readings must be recorded continuously by high speed recorder

Advantages of stopped flow method over continuous flow method • Volume of reagent used is very little • A permanent recorder can record the progress of the reactions as a wide range • This method is not affected by the rate and characters of the flow

Disadvanges • Less sensitive than continuous flow method • There are fewer convenient methods of observations

2. Relaxation Method • Flow methods are not suitable for the study of reactions whose half lives are smaller than 1 ms. So competition methods are used. • The reaction is first allowed to reach equilibrium and the disturbed in some way like changing of temperature or pressure its approach to a new equilibrium by using high speed techniques, the relaxation time of the process can be calculated

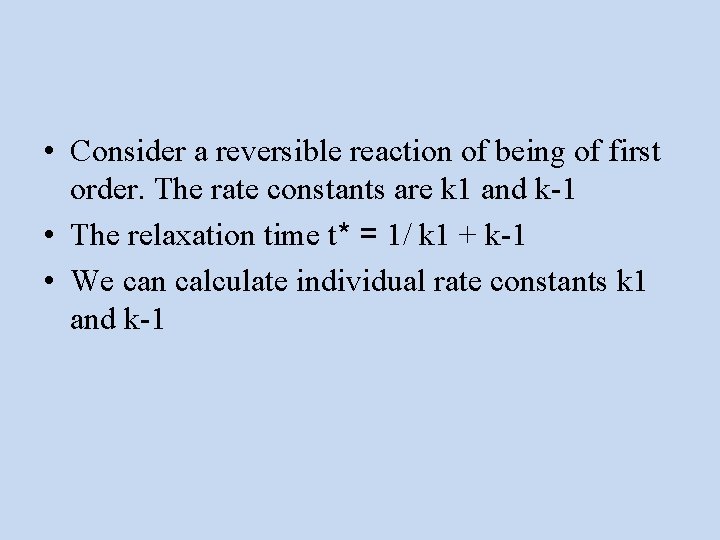
• Consider a reversible reaction of being of first order. The rate constants are k 1 and k-1 • The relaxation time t* = 1/ k 1 + k-1 • We can calculate individual rate constants k 1 and k-1

3. Shock Method • Principle • Shock wave pass through a long metal tube • Temperature of the gas increases and the reaction occurs • Metal tune of 6 inches in diameter and 20 feat long. A thin diaphram divides the tube into high pressure and low pressure end. • The reaction system is in the low pressure compartment

• The diaphram burst by mechanical means and the shock wave moves along the tube. • As the wave passes the gas is rapidly raised to a very high temperature and the reaction can be studied by high speed observations at various points along the tube. • Eg. Thermal decomposition of N 2 O 4 to NO 2

4. Flash Photolysis • Light flash of very high intensity and very short duration ( appoximately 10 -6 S) • Atoms, free radicals and excited species are produced and are undergo further reactions • A series of spectra taken after various intervals gives information about the course of reaction

5. Pulsed Laser Method • LASER- Light amplification by stimulated emission of radiation • MASER- Microwave amplification by stimulated emission of radiation • In a Laser beam a photon released by one atom, interact with another atom having population inversion and stimulate the release of another photon.

• By the chemical reaction of radiation several transient species and stable end products are produced. • Stable end products can be analysed by steadystate radiolysis • Kinetics of formation and decay of short lived intermediates and their spectral properties are investigated by pulse radiolysis coupled with fast detection techniques