Kinetics of Disinfection Ideally All cells equally mixed
- Slides: 11

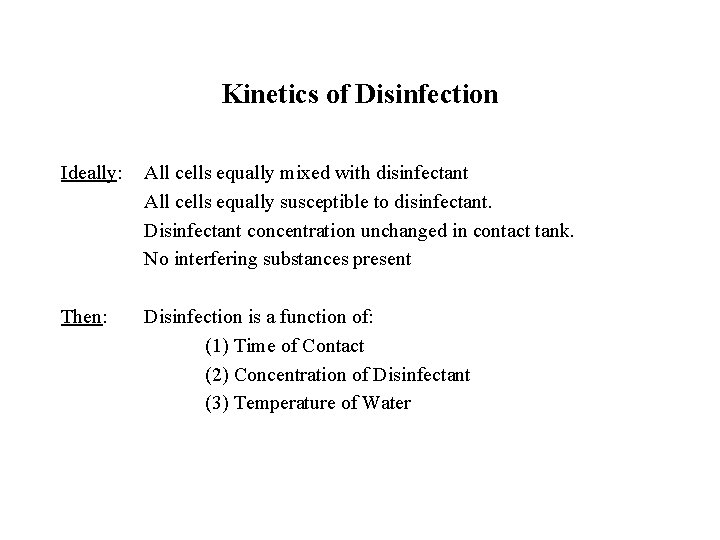
Kinetics of Disinfection Ideally: All cells equally mixed with disinfectant All cells equally susceptible to disinfectant. Disinfectant concentration unchanged in contact tank. No interfering substances present Then: Disinfection is a function of: (1) Time of Contact (2) Concentration of Disinfectant (3) Temperature of Water

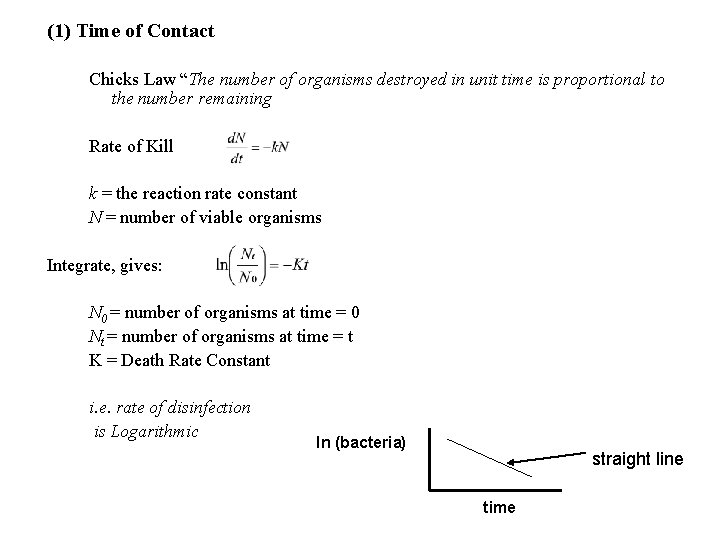
(1) Time of Contact Chicks Law “The number of organisms destroyed in unit time is proportional to the number remaining Rate of Kill k = the reaction rate constant N = number of viable organisms Integrate, gives: N 0 = number of organisms at time = 0 Nt = number of organisms at time = t K = Death Rate Constant i. e. rate of disinfection is Logarithmic ln (bacteria) straight line time

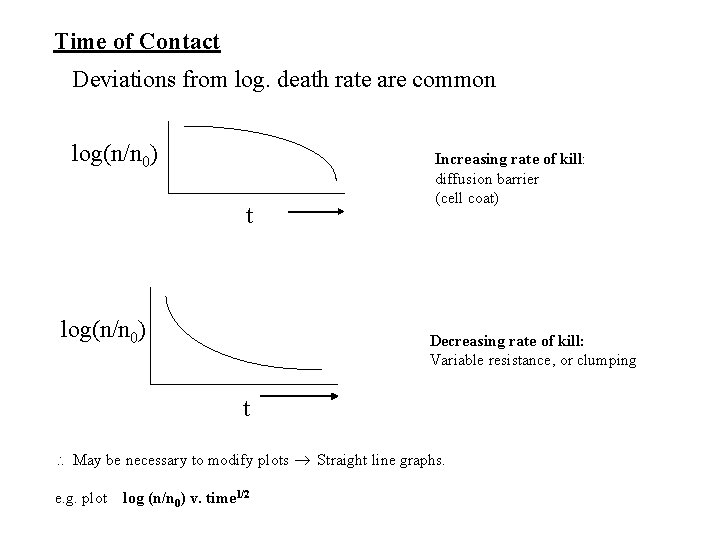
Time of Contact Deviations from log. death rate are common log(n/n 0) t log(n/n 0) Increasing rate of kill: diffusion barrier (cell coat) Decreasing rate of kill: Variable resistance, or clumping t May be necessary to modify plots Straight line graphs. e. g. plot log (n/n 0) v. time 1/2

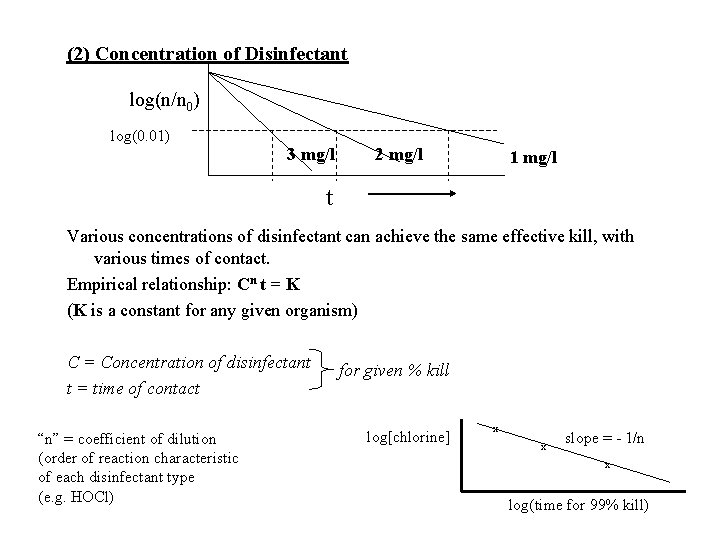
(2) Concentration of Disinfectant log(n/n 0) log(0. 01) 3 mg/l 2 mg/l 1 mg/l t Various concentrations of disinfectant can achieve the same effective kill, with various times of contact. Empirical relationship: Cn t = K (K is a constant for any given organism) C = Concentration of disinfectant t = time of contact “n” = coefficient of dilution (order of reaction characteristic of each disinfectant type (e. g. HOCl) for given % kill log[chlorine] x x slope = - 1/n x log(time for 99% kill)

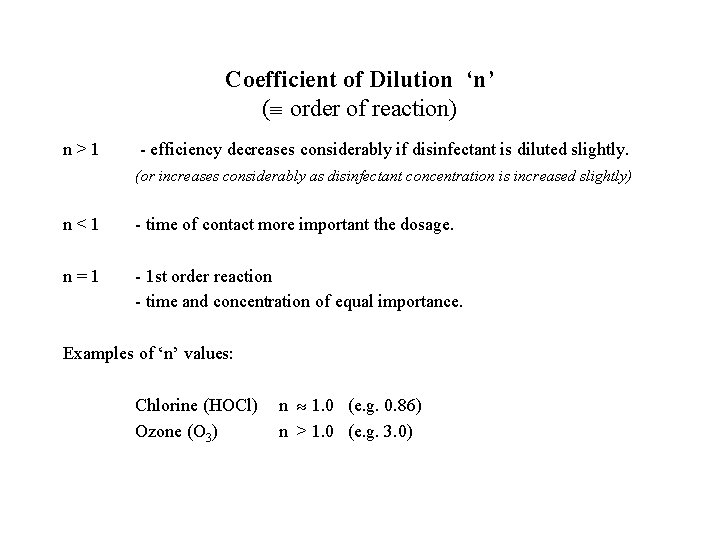
Coefficient of Dilution ‘n’ ( order of reaction) n>1 - efficiency decreases considerably if disinfectant is diluted slightly. (or increases considerably as disinfectant concentration is increased slightly) n<1 - time of contact more important the dosage. n=1 - 1 st order reaction - time and concentration of equal importance. Examples of ‘n’ values: Chlorine (HOCl) Ozone (O 3) n 1. 0 (e. g. 0. 86) n > 1. 0 (e. g. 3. 0)

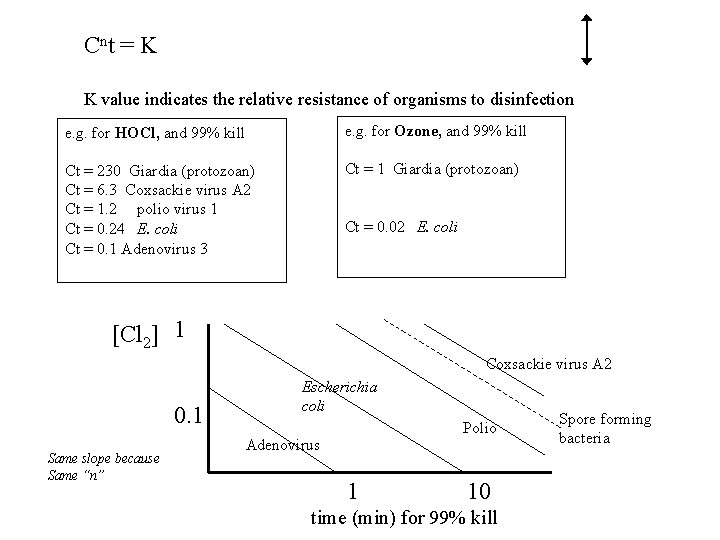
C nt = K K value indicates the relative resistance of organisms to disinfection e. g. for HOCl, and 99% kill e. g. for Ozone, and 99% kill Ct = 230 Giardia (protozoan) Ct = 6. 3 Coxsackie virus A 2 Ct = 1. 2 polio virus 1 Ct = 0. 24 E. coli Ct = 0. 1 Adenovirus 3 Ct = 1 Giardia (protozoan) Ct = 0. 02 E. coli [Cl 2] 1 Coxsackie virus A 2 0. 1 Same slope because Same “n” Escherichia coli Polio Adenovirus 1 10 time (min) for 99% kill Spore forming bacteria

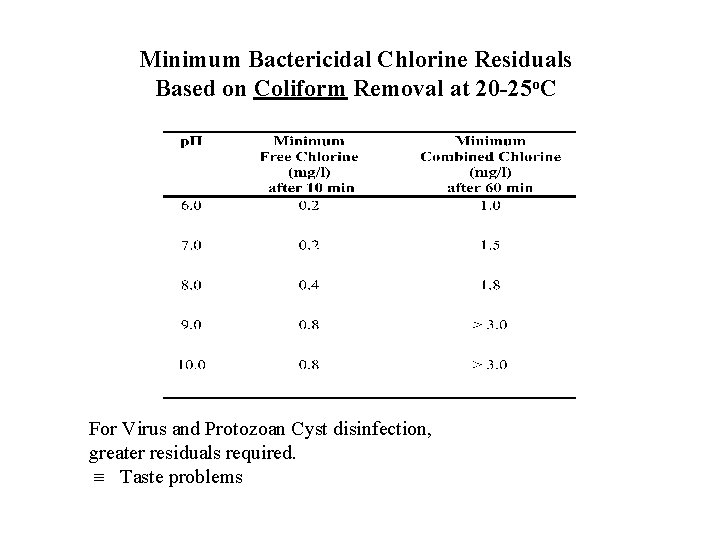
Minimum Bactericidal Chlorine Residuals Based on Coliform Removal at 20 -25 o. C For Virus and Protozoan Cyst disinfection, greater residuals required. Taste problems

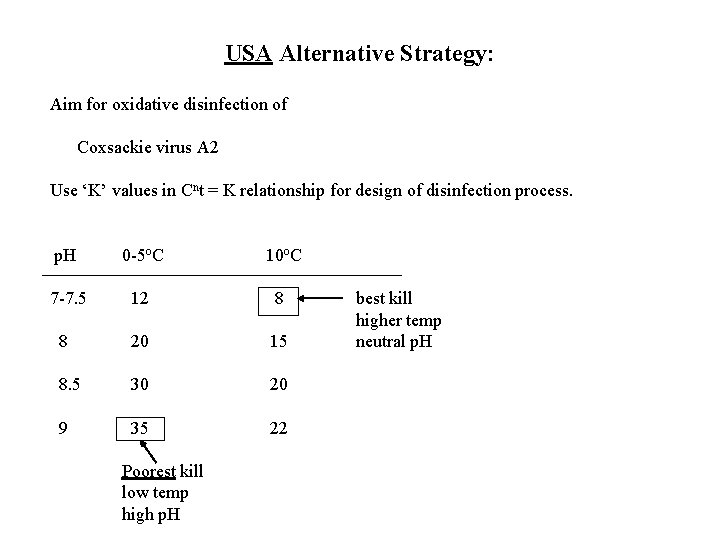
USA Alternative Strategy: Aim for oxidative disinfection of Coxsackie virus A 2 Use ‘K’ values in Cnt = K relationship for design of disinfection process. p. H 0 -5 o. C 10 o. C 7 -7. 5 12 8 8 20 15 8. 5 30 20 9 35 22 Poorest kill low temp high p. H best kill higher temp neutral p. H

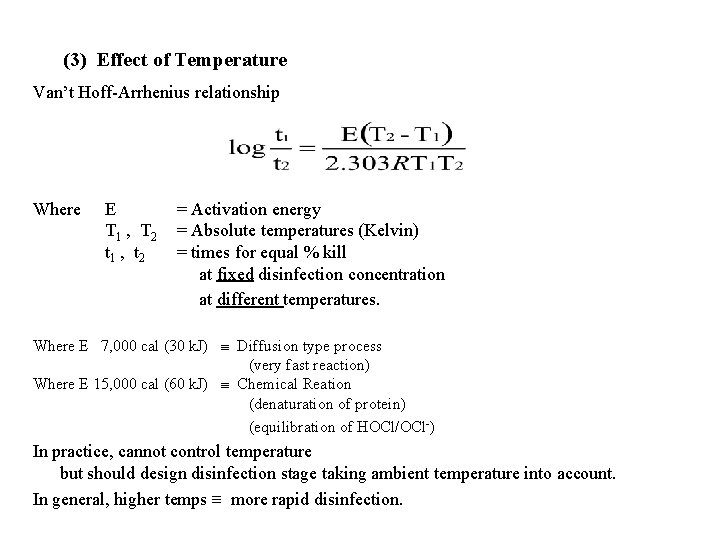
(3) Effect of Temperature Van’t Hoff-Arrhenius relationship Where E T 1 , T 2 t 1 , t 2 = Activation energy = Absolute temperatures (Kelvin) = times for equal % kill at fixed disinfection concentration at different temperatures. Where E 7, 000 cal (30 k. J) Diffusion type process (very fast reaction) Where E 15, 000 cal (60 k. J) Chemical Reation (denaturation of protein) (equilibration of HOCl/OCl-) In practice, cannot control temperature but should design disinfection stage taking ambient temperature into account. In general, higher temps more rapid disinfection.

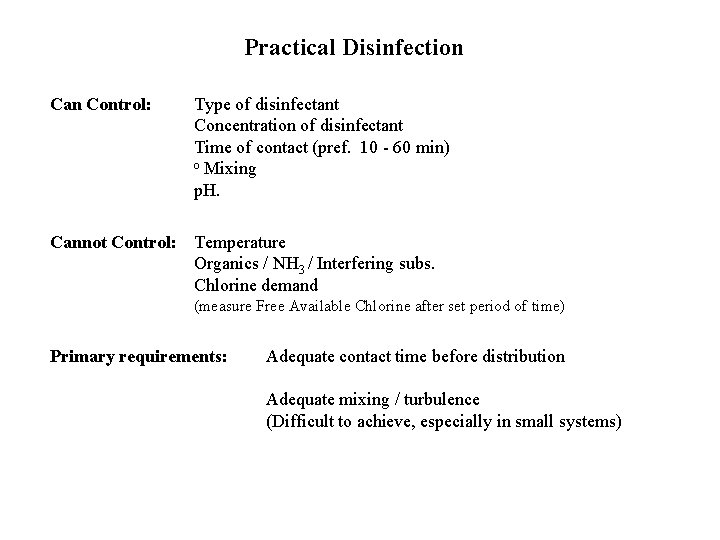
Practical Disinfection Can Control: Type of disinfectant Concentration of disinfectant Time of contact (pref. 10 - 60 min) o Mixing p. H. Cannot Control: Temperature Organics / NH 3 / Interfering subs. Chlorine demand (measure Free Available Chlorine after set period of time) Primary requirements: Adequate contact time before distribution Adequate mixing / turbulence (Difficult to achieve, especially in small systems)

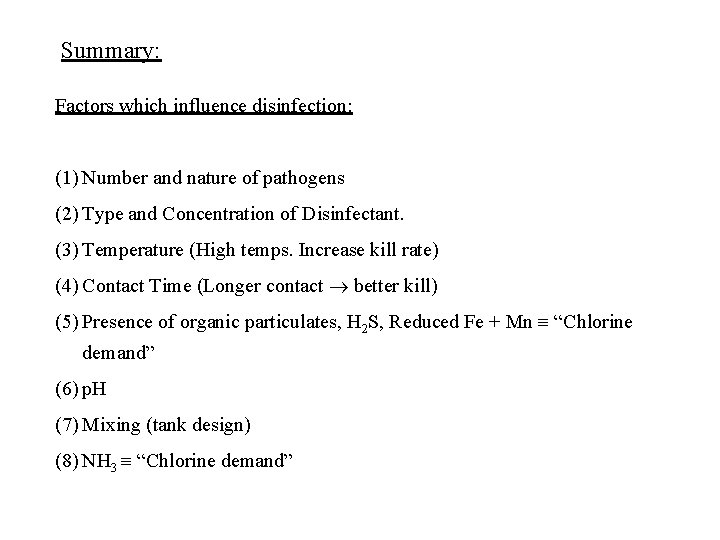
Summary: Factors which influence disinfection: (1) Number and nature of pathogens (2) Type and Concentration of Disinfectant. (3) Temperature (High temps. Increase kill rate) (4) Contact Time (Longer contact better kill) (5) Presence of organic particulates, H 2 S, Reduced Fe + Mn “Chlorine demand” (6) p. H (7) Mixing (tank design) (8) NH 3 “Chlorine demand”
 I wonder by my troth what thou and i
I wonder by my troth what thou and i All languages are equally complex
All languages are equally complex Optional and mandatory relationship in erd
Optional and mandatory relationship in erd Ideally insurable loss exposure
Ideally insurable loss exposure Ideally polarizable electrode
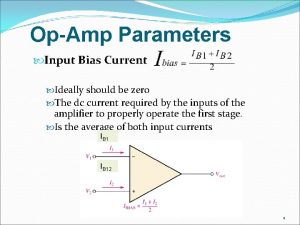
Ideally polarizable electrode Ideally the input bias current should be__________ . *
Ideally the input bias current should be__________ . * Measurements system analysis
Measurements system analysis Double pot method of disinfection
Double pot method of disinfection Electrostatic disinfection chelsea
Electrostatic disinfection chelsea Double pot method for disinfection of wells
Double pot method for disinfection of wells Low level disinfectant
Low level disinfectant Dry mist disinfection machine
Dry mist disinfection machine