Kinetics Lesson 3 Collision Theory The Collision Theory














- Slides: 19

Kinetics Lesson 3 Collision Theory

The Collision Theory Link to Simulation of Molecular Motion 1. Matter is moving particles. 2. Temperature increases- particles move faster -more collisions -more collision energy. 3. Chemical reactions -bonds break -new bonds form 4. Collisions provide the energy.

Collision Theory You need a collision to have a reaction. Collisions provide the energy required to break bonds. Most collisions are not successful

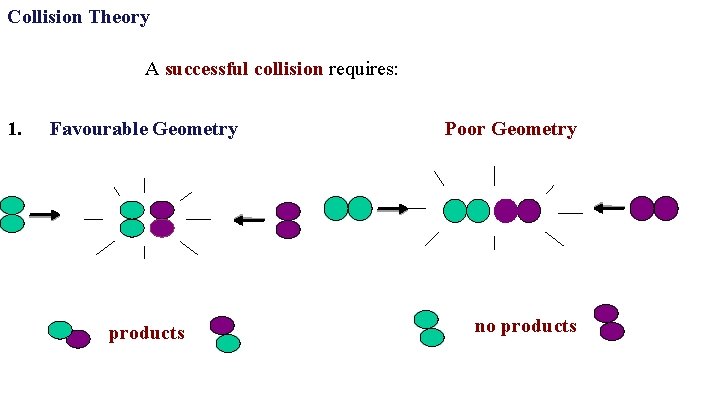
Collision Theory A successful collision requires: 1. Favourable Geometry products Poor Geometry no products

2. Sufficient Energy to break the chemical bonds Activation energy is the minimum amount of energy required for a successful collision.

The Collision Theory can be used to explain how the rate of a reaction can be changed. Reaction rates can increase due to 1. More collisions 2. Harder collisions- greater collision energy 3. Lower activation energy or Ea- low energy collisions are more effective. And that’s it!

The Collision Theory can be used to explain how the rate of a reaction can be changed. 1. Increasing the temperature increases the rate because there are: More collisions Harder collisions

2. Increasing the reactant concentration increases the rate because there are: More frequent collisions

The Collision Theory can be used to explain how the rate of a reaction can be changed. 3. Adding a catalyst Lowers the activation energy or Ea- allowing low energy collisions to be successful The catalyst KI is added to H 2 O 2, food colouring, and dishwashing detergent. The O 2 produced makes foam. Movie

The Collision Theory can be used to explain how the rate of a reaction can be changed. 4. Changing the nature of the reactant for a more reactive chemical increases the rate Lower activation energy or Ea- allowing low energy collisions to be successful

The Collision Theory can be used to explain how the rate of a reaction can be changed. 5. Increasing the surface area of a solid reactant increases the rate because: More frequent collisions

Explain each Scenario Using the Collision Theory 1. A balloon full of H 2 and O 2 do not react at room temperature. Ea is too high for the room temperature collisions A small spark ignites causes an explosion. The spark provides the Ea and it explodes because it is exothermic

Explain each Scenario Using the Collision Theory 2. A candle does not burn at room temperature Ea is too high for the room temperature collisions A match causes the candle to burn The match provides the Ea The candle continues to burn It burns because it is exothermic

Explain each Scenario Using the Collision Theory 3. H 2 O 2 decomposes very slowly at room temperature. 2 H 2 O 2(aq) → O 2(g) + 2 H 2 O(l) KI increases the reaction rate dramatically. KI is a catalyst as it is not a reactant and it speeds up the rate. Lowers the Ea- allows low energy collisions to be successful

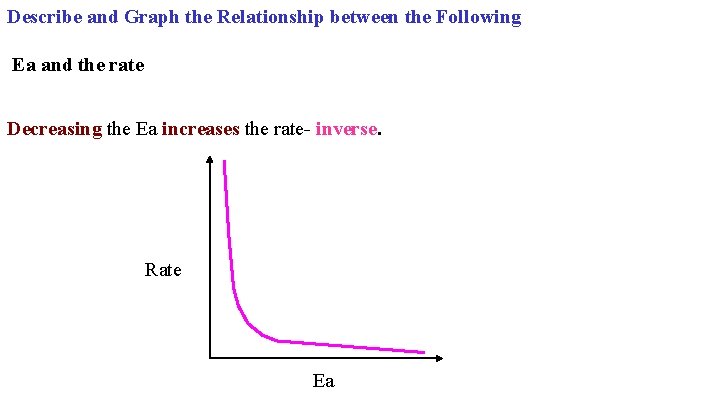
Describe and Graph the Relationship between the Following Ea and the rate Decreasing the Ea increases the rate- inverse. Rate Ea

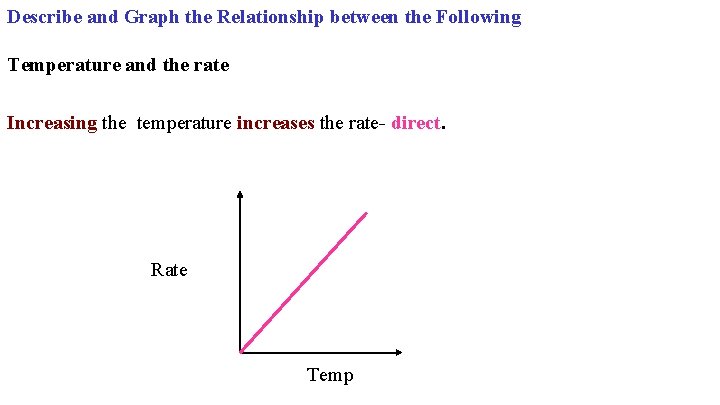
Describe and Graph the Relationship between the Following Temperature and the rate Increasing the temperature increases the rate- direct. Rate Temp

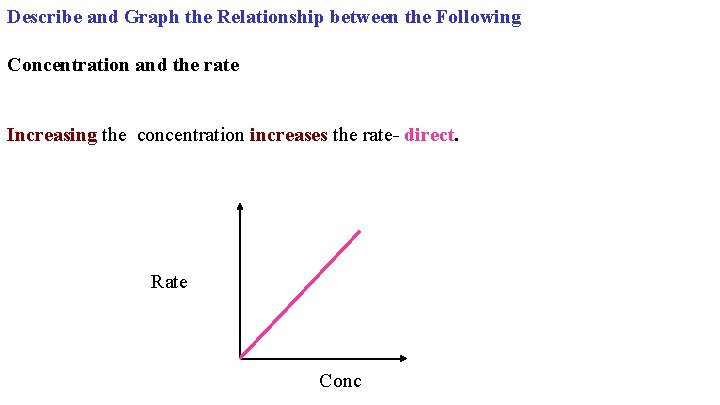
Describe and Graph the Relationship between the Following Concentration and the rate Increasing the concentration increases the rate- direct. Rate Conc

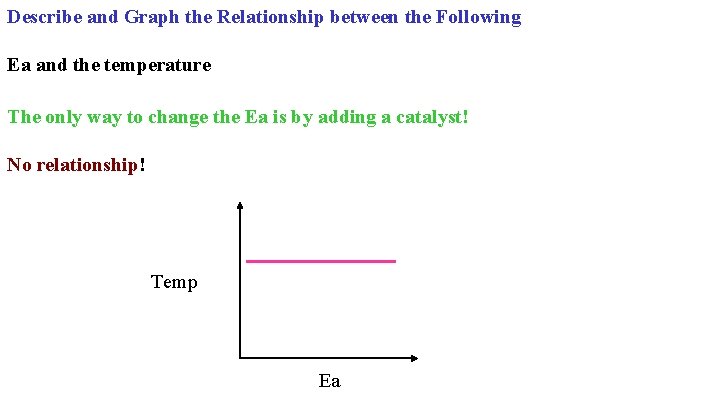
Describe and Graph the Relationship between the Following Ea and the temperature The only way to change the Ea is by adding a catalyst! No relationship! Temp Ea

Which factors increase the percentage of successful collisions? I. Increasing temperature II. Increasing concentration III. Increasing surface area IV. Adding a catalyst