Kinetics How fast does a reaction event occur
![Concentration and Time-1 st order reactions Rate = k[A] Integrated rate law We can Concentration and Time-1 st order reactions Rate = k[A] Integrated rate law We can](https://slidetodoc.com/presentation_image/bff1d03ade2d14f2efcc1af83b1cbc0e/image-5.jpg)
![Concentration and Time-1 st order reactions A plot of ln[A]t versus t is a Concentration and Time-1 st order reactions A plot of ln[A]t versus t is a](https://slidetodoc.com/presentation_image/bff1d03ade2d14f2efcc1af83b1cbc0e/image-8.jpg)


- Slides: 22

Kinetics How fast does a reaction (event) occur? Reaction rates are controlled by: • Nature of reactants • Ability of reactants to meet • Concentration of reactants • Temperature • Presence of a catalyst Rate of pay = € 10/hour UNITS: mol/L x 1/s =mol. L-1. s-1 or M. s-1

Kinetics Change of reaction rate with time Concentration and rate A + B products In general it is found that: rate [A]m[B]n The values of the exponents, m and n, must be determined empirically (by experiment). We can replace by = if we introduce a rate constant, k. Rate = k [A]m[B]n This expression is the rate law

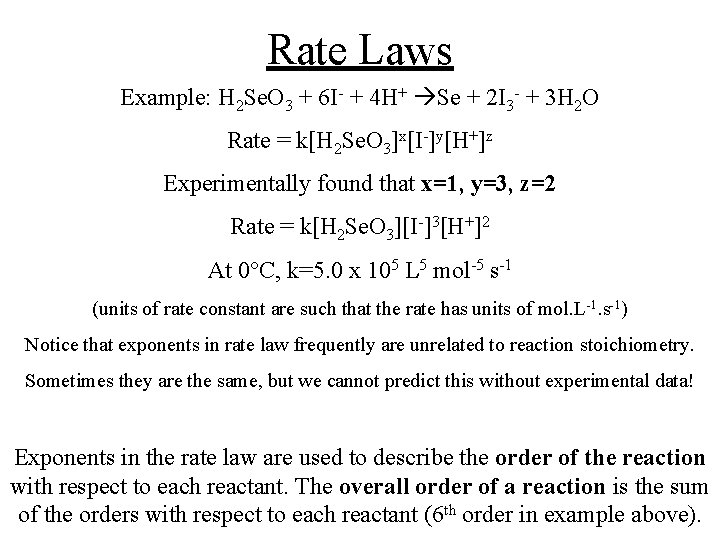
Rate Laws Example: H 2 Se. O 3 + 6 I- + 4 H+ Se + 2 I 3 - + 3 H 2 O Rate = k[H 2 Se. O 3]x[I-]y[H+]z Experimentally found that x=1, y=3, z=2 Rate = k[H 2 Se. O 3][I-]3[H+]2 At 0 C, k=5. 0 x 105 L 5 mol-5 s-1 (units of rate constant are such that the rate has units of mol. L-1. s-1) Notice that exponents in rate law frequently are unrelated to reaction stoichiometry. Sometimes they are the same, but we cannot predict this without experimental data! Exponents in the rate law are used to describe the order of the reaction with respect to each reactant. The overall order of a reaction is the sum of the orders with respect to each reactant (6 th order in example above).

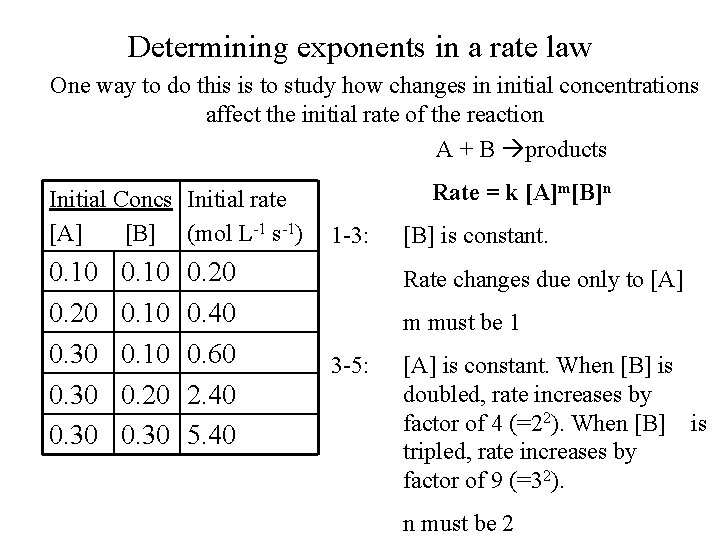
Determining exponents in a rate law One way to do this is to study how changes in initial concentrations affect the initial rate of the reaction A + B products Initial Concs Initial rate [A] [B] (mol L-1 s-1) 0. 10 0. 20 0. 30 0. 20 0. 40 0. 60 2. 40 5. 40 Rate = k [A]m[B]n 1 -3: [B] is constant. Rate changes due only to [A] m must be 1 3 -5: [A] is constant. When [B] is doubled, rate increases by factor of 4 (=22). When [B] is tripled, rate increases by factor of 9 (=32). n must be 2
![Concentration and Time1 st order reactions Rate kA Integrated rate law We can Concentration and Time-1 st order reactions Rate = k[A] Integrated rate law We can](https://slidetodoc.com/presentation_image/bff1d03ade2d14f2efcc1af83b1cbc0e/image-5.jpg)
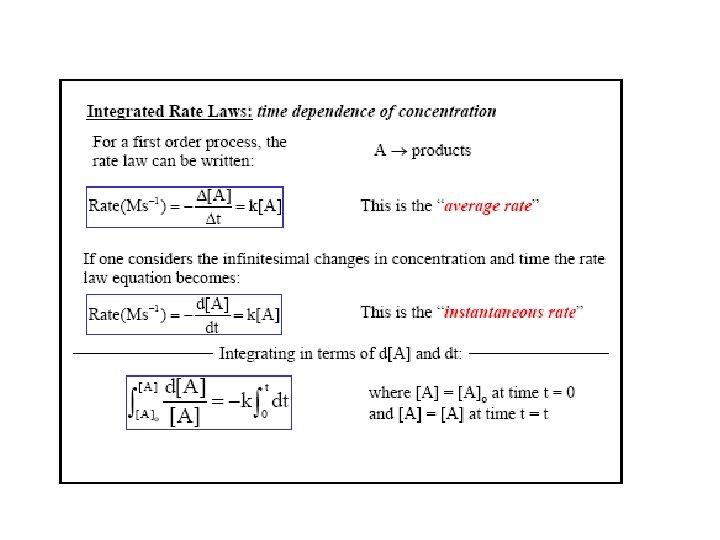
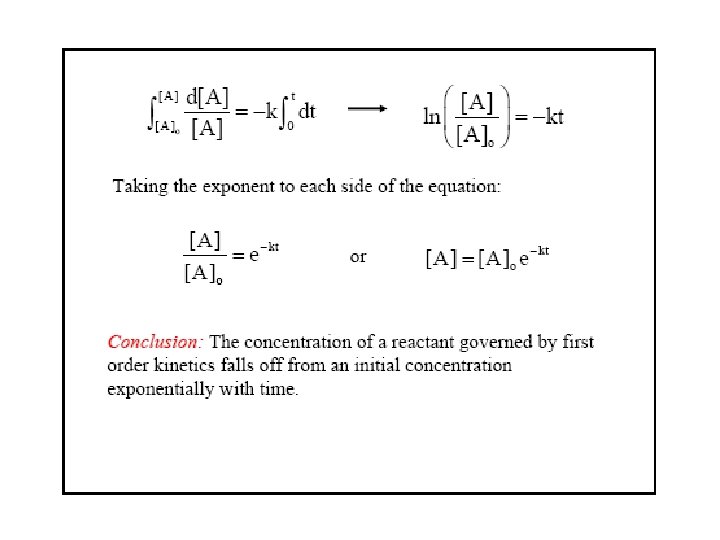
Concentration and Time-1 st order reactions Rate = k[A] Integrated rate law We can show that A plot of ln[A]t versus t is a straight line y = mx + c with slope -k and y intercept ln[A]0.


![Concentration and Time1 st order reactions A plot of lnAt versus t is a Concentration and Time-1 st order reactions A plot of ln[A]t versus t is a](https://slidetodoc.com/presentation_image/bff1d03ade2d14f2efcc1af83b1cbc0e/image-8.jpg)
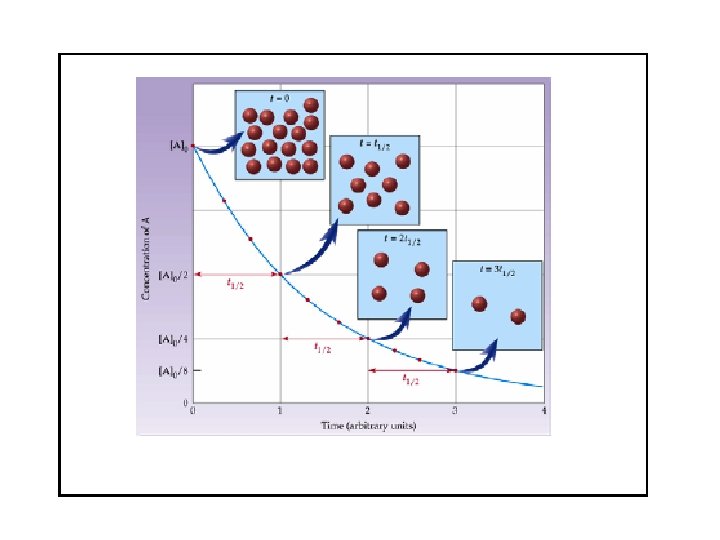
Concentration and Time-1 st order reactions A plot of ln[A]t versus t is a straight line with slope -k and y intercept ln[A]0. Half-life: time required for half of initial concentration of reactant to disappear. Set [A]t = ½[A]0 t 1/2 = ln 2/k



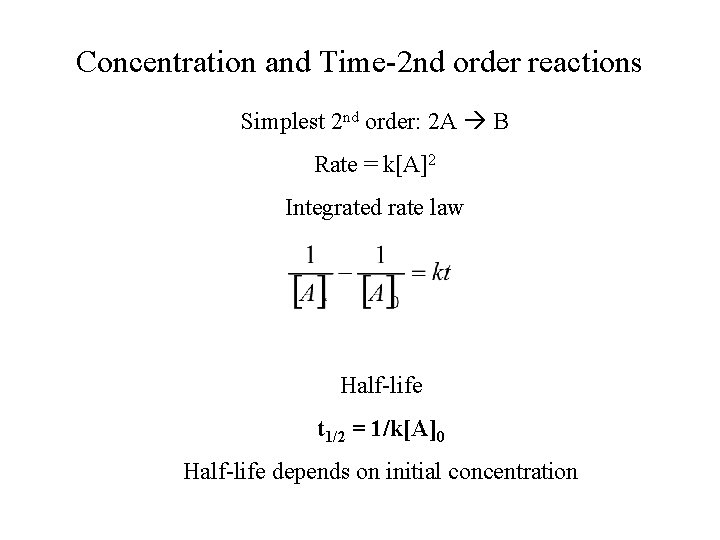
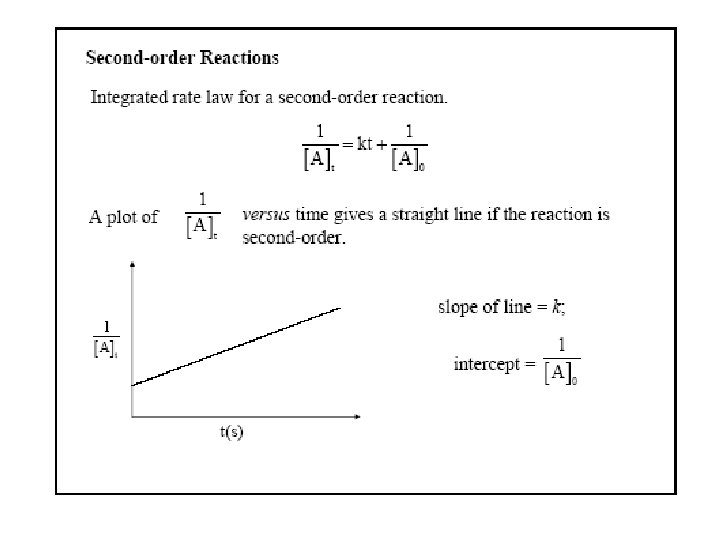
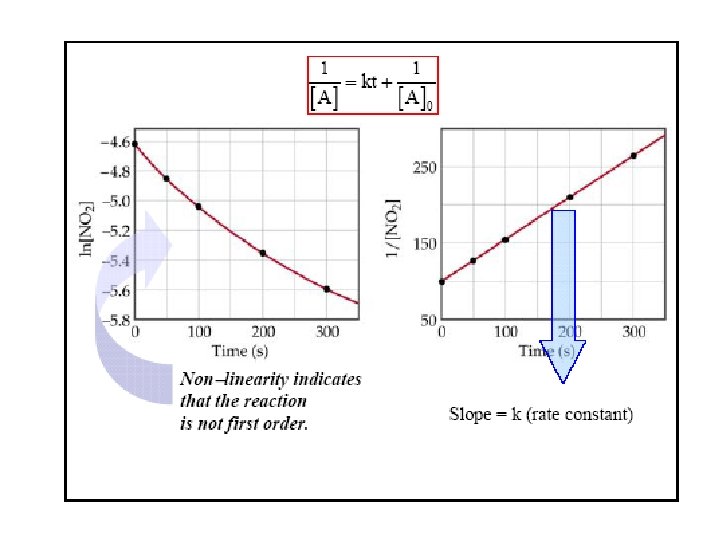
Concentration and Time-2 nd order reactions Simplest 2 nd order: 2 A B Rate = k[A]2 Integrated rate law Half-life t 1/2 = 1/k[A]0 Half-life depends on initial concentration



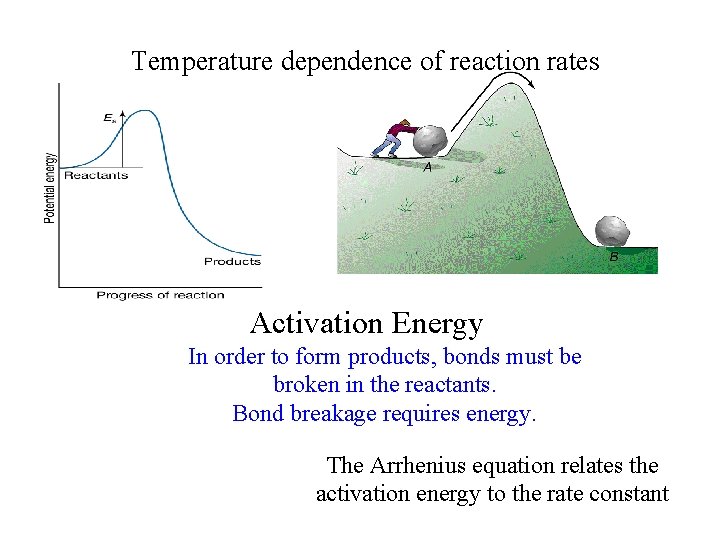
Temperature dependence of reaction rates Activation Energy In order to form products, bonds must be broken in the reactants. Bond breakage requires energy. The Arrhenius equation relates the activation energy to the rate constant

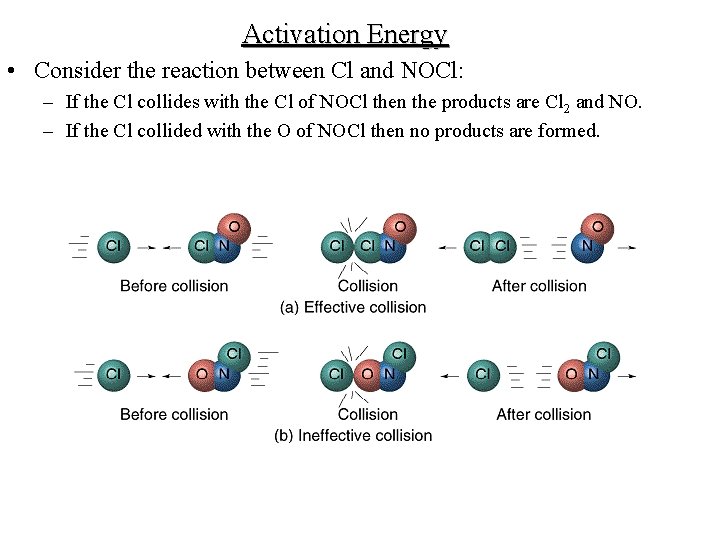
Activation Energy • Consider the reaction between Cl and NOCl: – If the Cl collides with the Cl of NOCl then the products are Cl 2 and NO. – If the Cl collided with the O of NOCl then no products are formed.

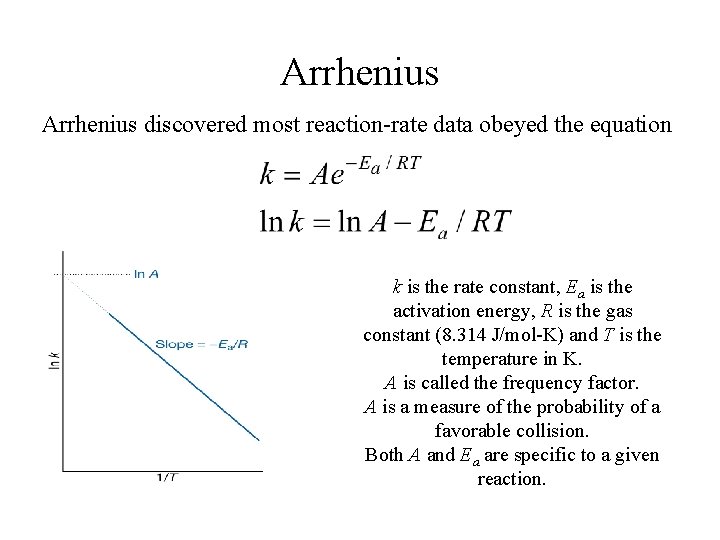
Arrhenius discovered most reaction-rate data obeyed the equation k is the rate constant, Ea is the activation energy, R is the gas constant (8. 314 J/mol-K) and T is the temperature in K. A is called the frequency factor. A is a measure of the probability of a favorable collision. Both A and Ea are specific to a given reaction.

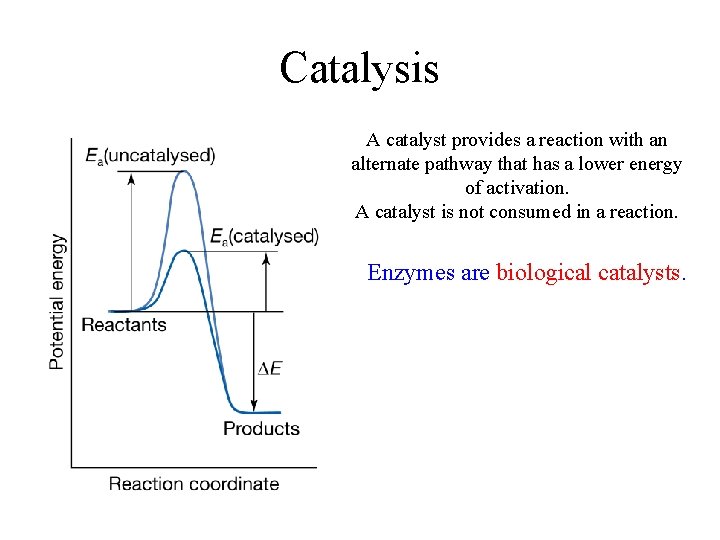
Catalysis A catalyst provides a reaction with an alternate pathway that has a lower energy of activation. A catalyst is not consumed in a reaction. Enzymes are biological catalysts.

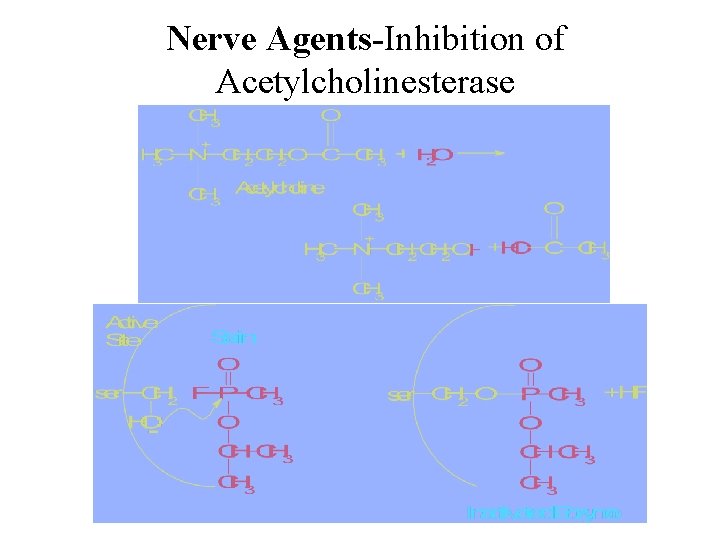
Nerve Agents-Inhibition of Acetylcholinesterase

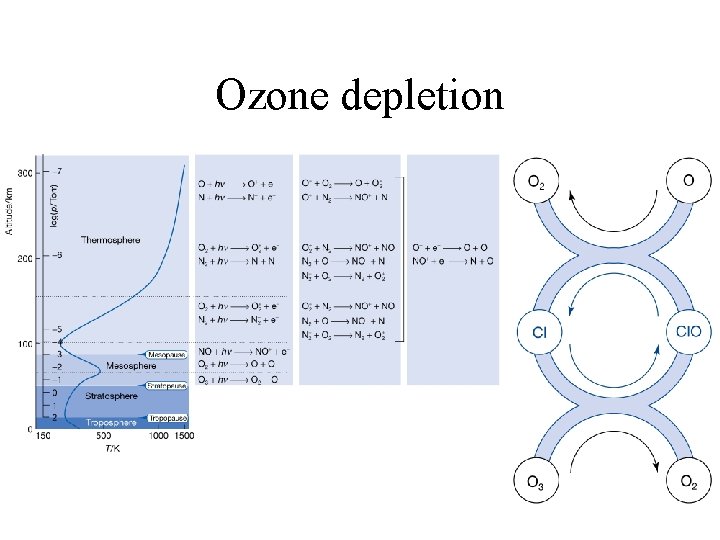
Ozone depletion

Radio-activity Unstable atomic nuclei may decay by emitting particles that are detected with special counters. Alpha, beta, and gamma emission are common types of radioactivity. In beta decay the emitted particles are electrons; in alpha decay they are helium nuclei, and in gamma decay they are high energy photons. Counters can be sensitive to either alpha, beta, or gamma-ray particles. The rubidium isotope 37 Rb 87 decays by beta emission to 38 Sr 87, a stable strontium nucleus: 37 Rb 87 38 Sr 87 + b. From the following experimental data, calculate (a) the rate constant and (b) the half -life of the rubidium isotope. From a 1. 00 g sample of Rb. Cl which is 27. 85% 37 Rb 87, an activity of 478 beta counts per second was found. The molecular weight of Rb. Cl is 120. 9 g mole-1.

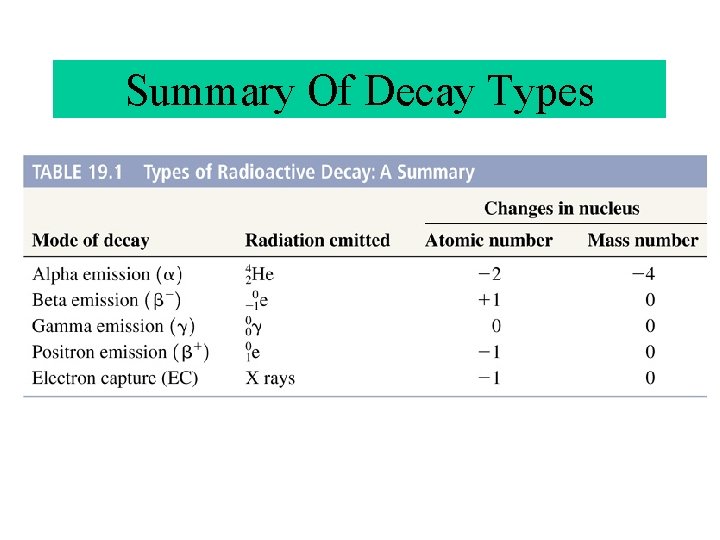
Summary Of Decay Types

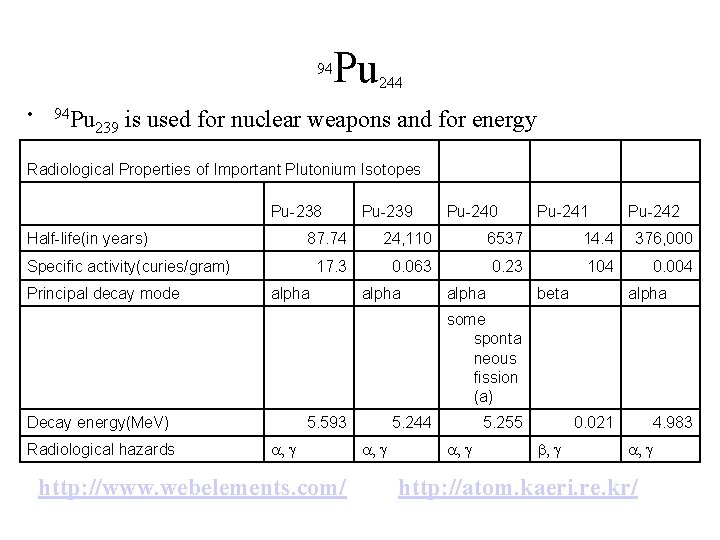
Pu 94 • 94 Pu 239 244 is used for nuclear weapons and for energy Radiological Properties of Important Plutonium Isotopes Pu-238 Half-life(in years) Pu-240 Pu-241 Pu-242 87. 74 24, 110 6537 14. 4 376, 000 17. 3 0. 063 0. 23 104 0. 004 Specific activity(curies/gram) Principal decay mode Pu-239 alpha beta alpha some sponta neous fission (a) Decay energy(Me. V) Radiological hazards 5. 593 a, g http: //www. webelements. com/ 5. 244 a, g 5. 255 a, g 0. 021 b, g 4. 983 a, g http: //atom. kaeri. re. kr/