Kinetics and Reactors Review CHBE 446 Chris Borowski

Kinetics and Reactors Review CHBE 446 Chris Borowski Yuri Choe Ethan Hamilton Andrew Lanouette Le An Nguyen Savannah Shaul

Kinetics
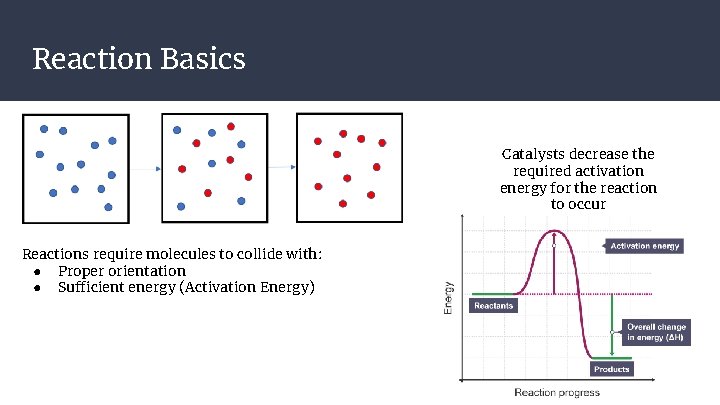
Reaction Basics Catalysts decrease the required activation energy for the reaction to occur Reactions require molecules to collide with: ● Proper orientation ● Sufficient energy (Activation Energy)
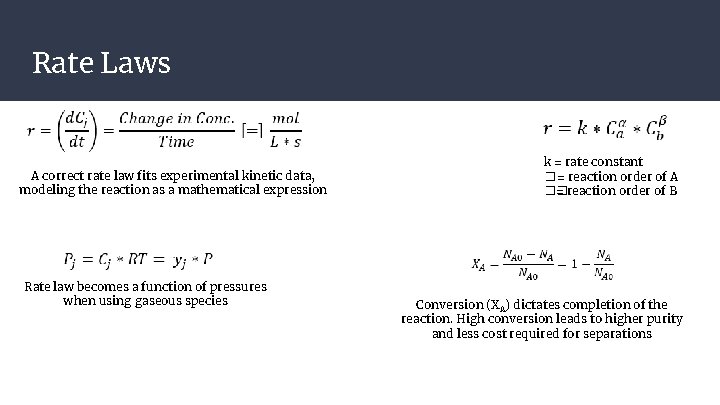
Rate Laws A correct rate law fits experimental kinetic data, modeling the reaction as a mathematical expression Rate law becomes a function of pressures when using gaseous species k = rate constant � = reaction order of A �� = reaction order of B Conversion (XA) dictates completion of the reaction. High conversion leads to higher purity and less cost required for separations
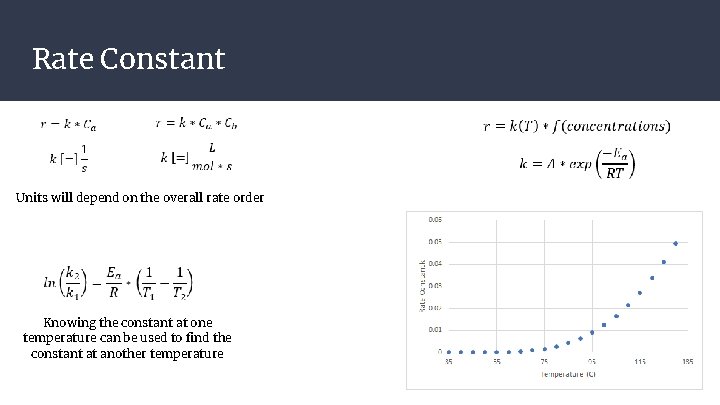
Rate Constant Units will depend on the overall rate order Knowing the constant at one temperature can be used to find the constant at another temperature

Reactor Types
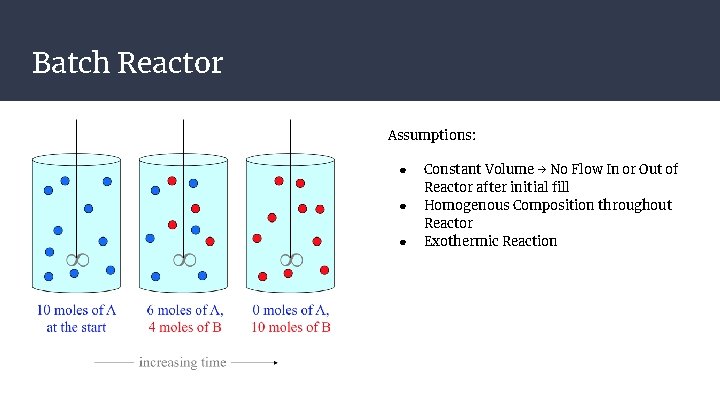
Batch Reactor Assumptions: ● ● ● Constant Volume → No Flow In or Out of Reactor after initial fill Homogenous Composition throughout Reactor Exothermic Reaction
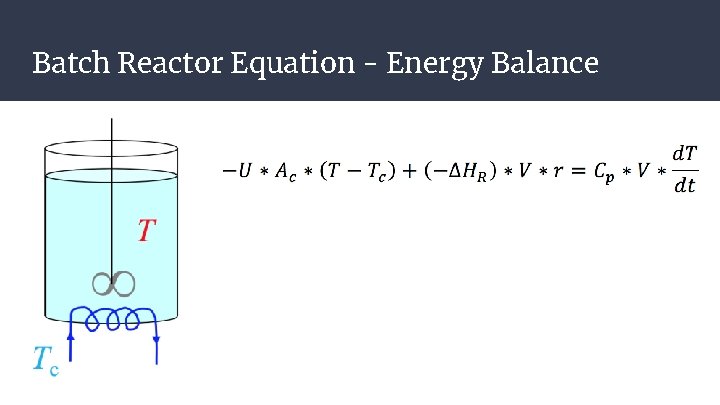
Batch Reactor Equation - Energy Balance
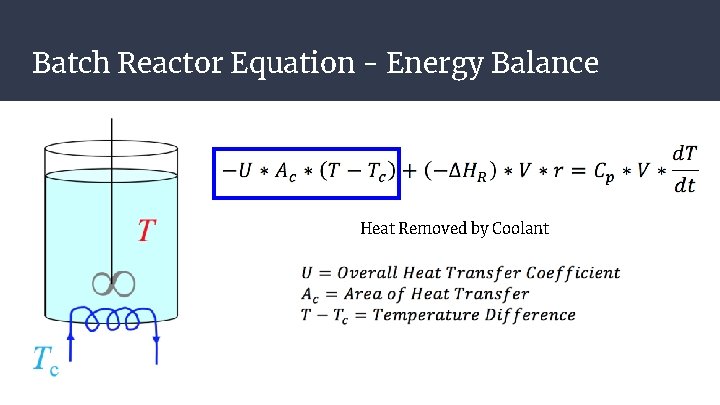
Batch Reactor Equation - Energy Balance Heat Removed by Coolant
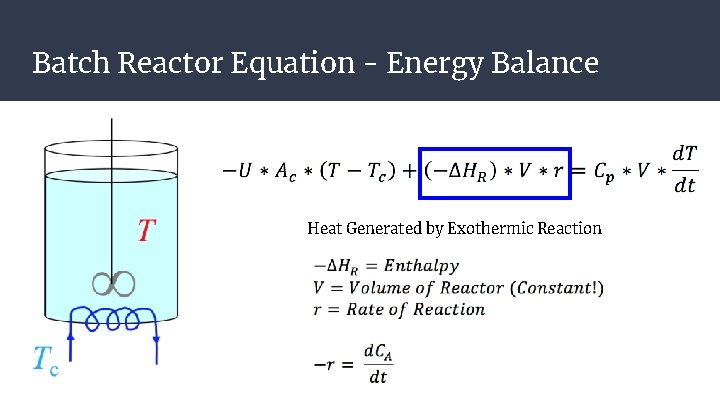
Batch Reactor Equation - Energy Balance Heat Generated by Exothermic Reaction
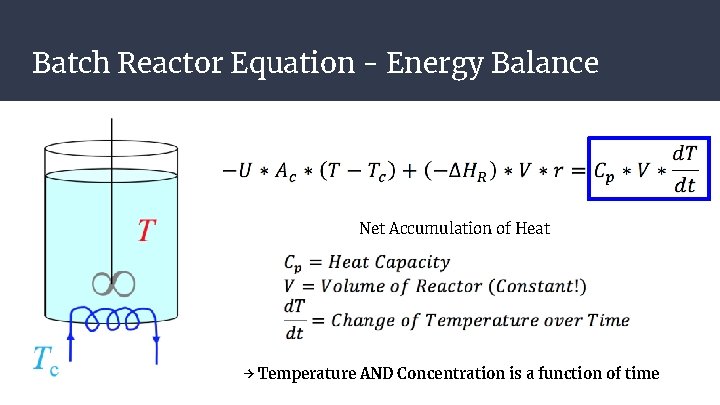
Batch Reactor Equation - Energy Balance Net Accumulation of Heat → Temperature AND Concentration is a function of time
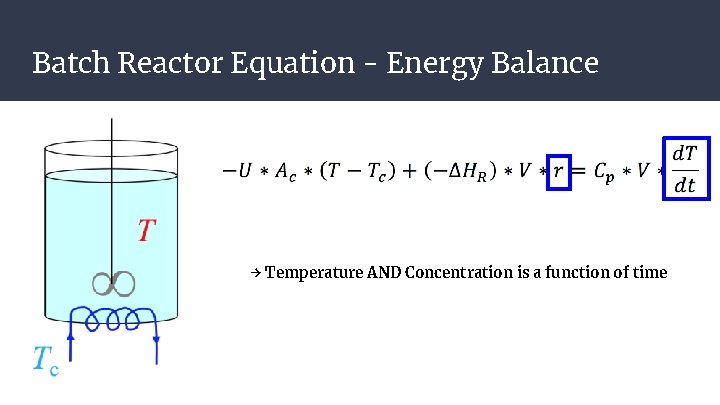
Batch Reactor Equation - Energy Balance → Temperature AND Concentration is a function of time
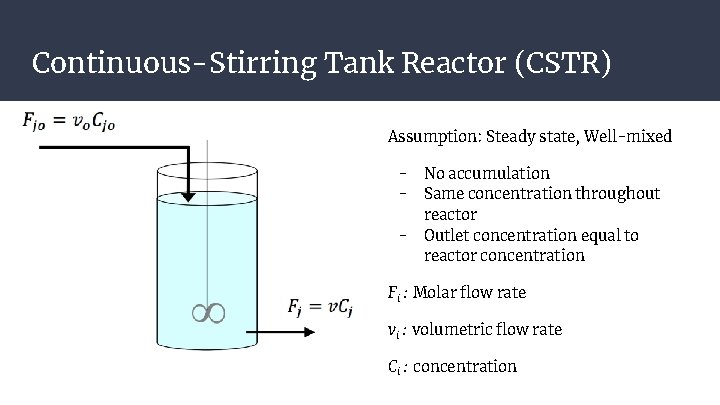
Continuous-Stirring Tank Reactor (CSTR) Assumption: Steady state, Well-mixed - No accumulation Same concentration throughout reactor Outlet concentration equal to reactor concentration F i : Molar flow rate v i : volumetric flow rate C i : concentration
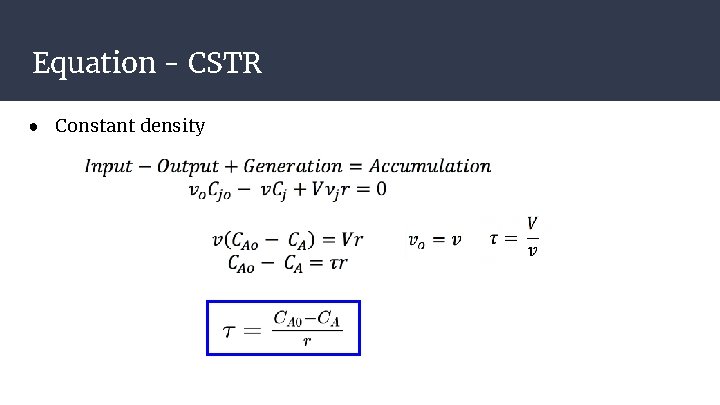
Equation - CSTR ● Constant density
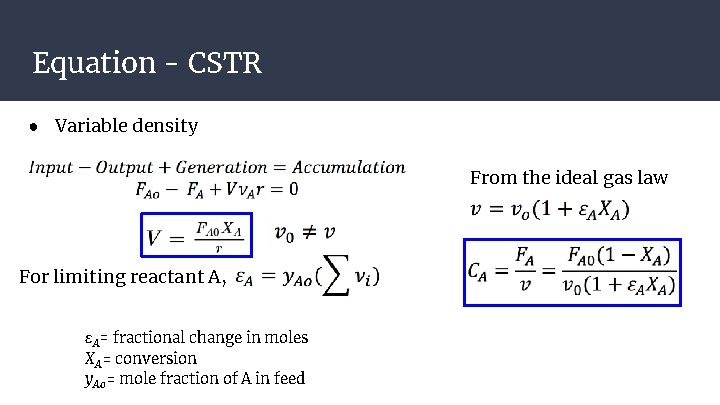
Equation - CSTR ● Variable density From the ideal gas law For limiting reactant A, εA= fractional change in moles X A= conversion y Ao = mole fraction of A in feed
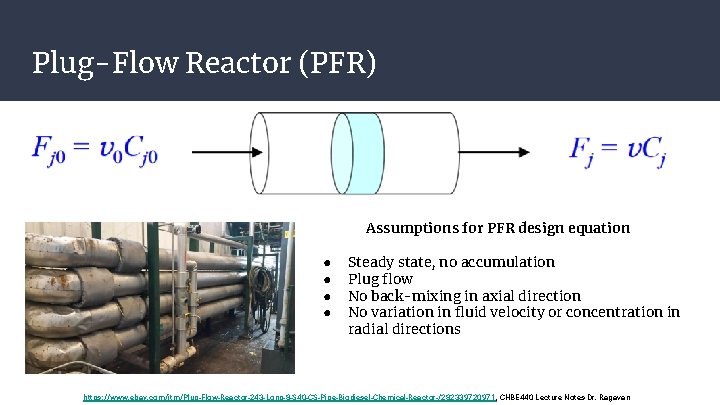
Plug-Flow Reactor (PFR) Assumptions for PFR design equation ● ● Steady state, no accumulation Plug flow No back-mixing in axial direction No variation in fluid velocity or concentration in radial directions https: //www. ebay. com/itm/Plug-Flow-Reactor-243 -Long-8 -S 40 -CS-Pipe-Biodiesel-Chemical-Reactor-/282339720971, CHBE 440 Lecture Notes Dr. Ragavan
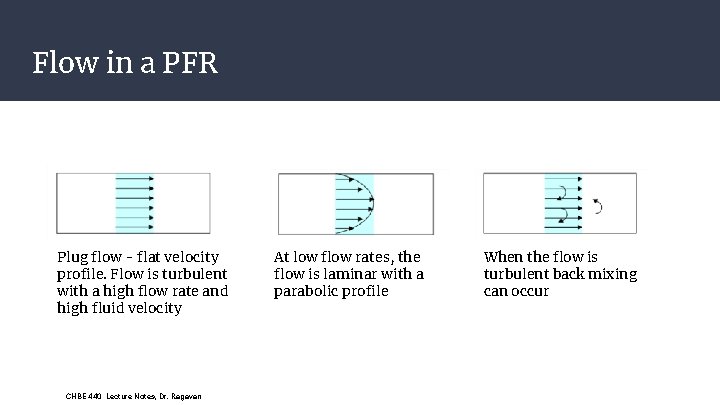
Flow in a PFR Plug flow - flat velocity profile. Flow is turbulent with a high flow rate and high fluid velocity CHBE 440 Lecture Notes, Dr. Ragavan At low flow rates, the flow is laminar with a parabolic profile When the flow is turbulent back mixing can occur
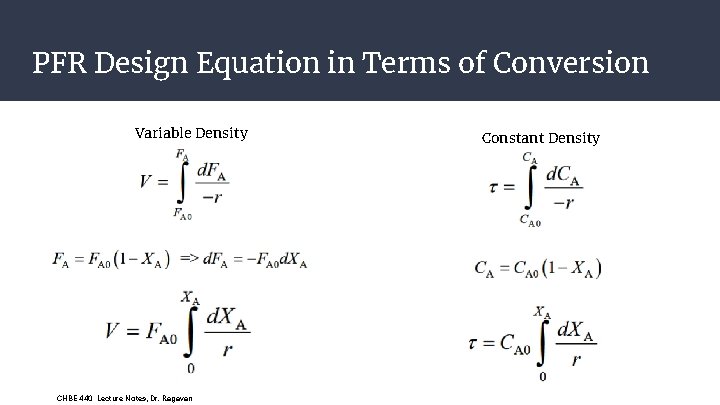
PFR Design Equation in Terms of Conversion Variable Density CHBE 440 Lecture Notes, Dr. Ragavan Constant Density
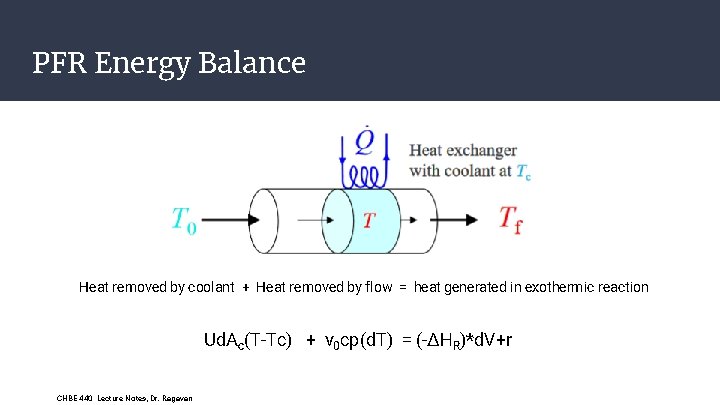
PFR Energy Balance Heat removed by coolant + Heat removed by flow = heat generated in exothermic reaction Ud. Ac(T-Tc) + v 0 cp(d. T) = (-ΔHR)*d. V+r CHBE 440 Lecture Notes, Dr. Ragavan

Reactor Applications
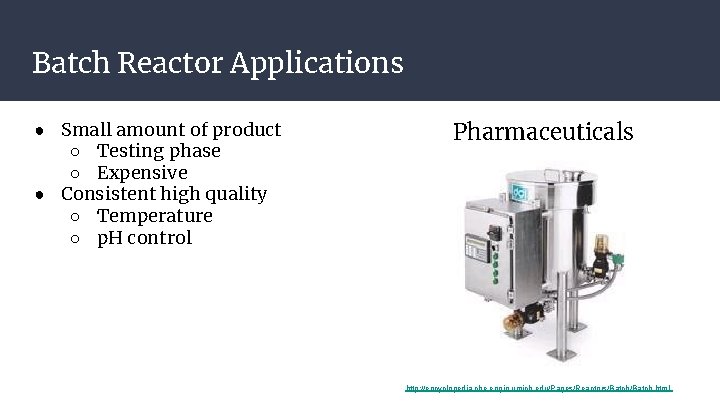
Batch Reactor Applications ● Small amount of product ○ Testing phase ○ Expensive ● Consistent high quality ○ Temperature ○ p. H control Pharmaceuticals http: //encyclopedia. che. engin. umich. edu/Pages/Reactors/Batch. html
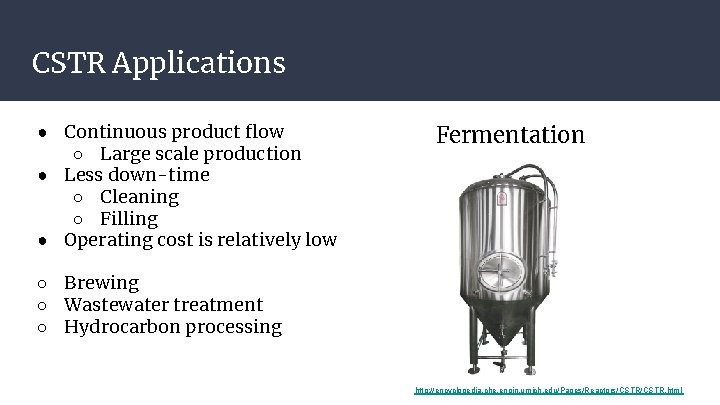
CSTR Applications ● Continuous product flow ○ Large scale production ● Less down-time ○ Cleaning ○ Filling ● Operating cost is relatively low Fermentation ○ Brewing ○ Wastewater treatment ○ Hydrocarbon processing http: //encyclopedia. che. engin. umich. edu/Pages/Reactors/CSTR. html
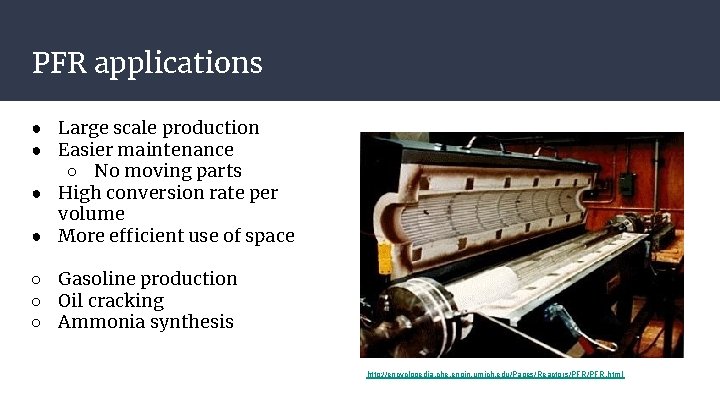
PFR applications ● Large scale production ● Easier maintenance ○ No moving parts ● High conversion rate per volume ● More efficient use of space ○ Gasoline production ○ Oil cracking ○ Ammonia synthesis http: //encyclopedia. che. engin. umich. edu/Pages/Reactors/PFR. html

Reactor Dangers and Safety
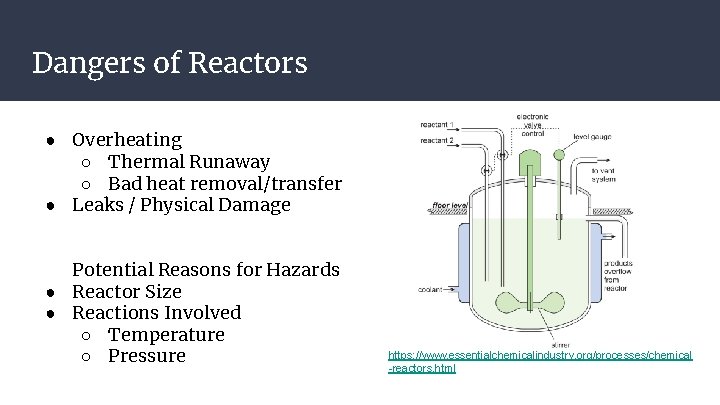
Dangers of Reactors ● Overheating ○ Thermal Runaway ○ Bad heat removal/transfer ● Leaks / Physical Damage Potential Reasons for Hazards ● Reactor Size ● Reactions Involved ○ Temperature ○ Pressure https: //www. essentialchemicalindustry. org/processes/chemical -reactors. html
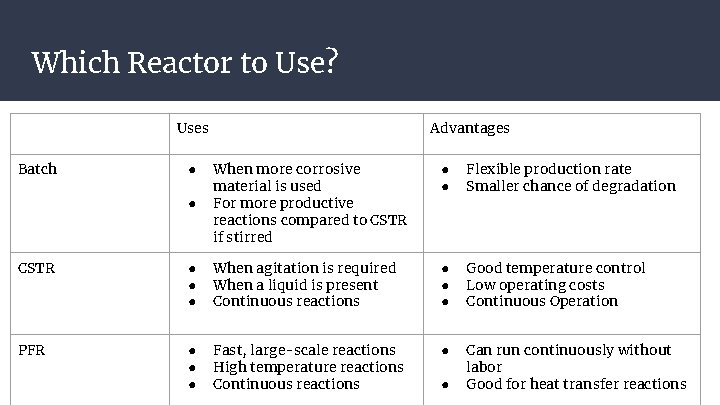
Which Reactor to Use? Uses Batch Advantages ● ● Flexible production rate Smaller chance of degradation ● When more corrosive material is used For more productive reactions compared to CSTR if stirred CSTR ● ● ● When agitation is required When a liquid is present Continuous reactions ● ● ● Good temperature control Low operating costs Continuous Operation PFR ● ● ● Fast, large-scale reactions High temperature reactions Continuous reactions ● Can run continuously without labor Good for heat transfer reactions ● ●
Additional Safety Precautions https: //www. osha. gov/SLTC/hazardoustoxicsubstances/control. html http: //umich. edu/~safeche/bowtie. html https: //oshmatters. wordpress. com/tag/physical-hazards-2/
- Slides: 27