Kinetic Theory of Mater States of matter Phase








- Slides: 14

- Kinetic Theory of Mater - States of matter - Phase changes - Classes of Matter - Properties of Matter - Conservation of Mass

�a theory that matter is composed of small particles, all in random motion. r e t t a M

� Solid � Liquid � Gas � Plasma

� Constant volume (fixed and wont change) � Constant shape � VERY slow movement of particles or only vibrate � Low energy (very little energy) � 4 types �Ionic �Covalent �Molecular �Metallic

Volume is constant (same) � Shape is NOT constant, it changes depending on the container � Particles move more quickly � Energy is higher than solid � Other � � Viscosity --The resistance of a liquid to flow is called its viscosity � Surface Tension -- The result of attraction between molecules of a liquid which causes the surface of the liquid to act as a thin elastic film under tension. Surface tension causes water to form spherical drops. � Vapor Pressure -- The pressure that a solid or liquid exerts when it is in equilibrium with its vapor at a given temperature. � Boiling Point -- when vapor pressure = atmospheric pressure.

� Not fixed Volume � Not fixed Shape, will take the shape of the container � Movement of particles is faster than a liquid � Higher energy than liquid or solid � Other- all the gases more or less obey the gas laws. The gas laws deal with how gases behave with respect to pressure, volume, temperature, and amount.

� � � � Not fixed volume Not fixed shape Fastest particle movement Highest energy Plasma is an ionized gas, or a gas into which sufficient energy is provided to free electrons from atoms or molecules and to allow both species, ions and electrons, to coexist. plasma is a cloud of protons, neutrons and electrons where all the electrons have come loose from their respective molecules and atoms, giving the plasma the ability to act as a whole rather than as a bunch of atoms. Plasmas are the most common state of matter in the universe comprising more than 99% of our visible universe and most of that not visible. On earth, plasma is naturally occurring in flames, lightning and the auroras. And can be seen in neon lights

�http: //phet. colorado. edu/en /simulation/states-of-matter (Phet Simulation) phet. colorado. edu

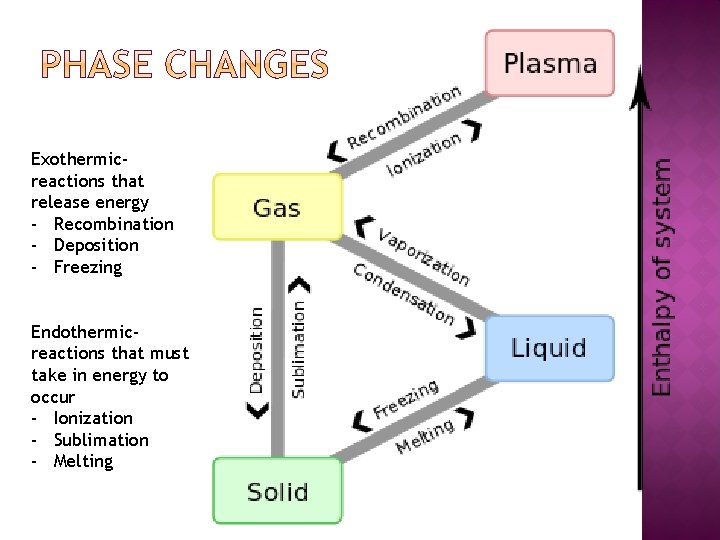
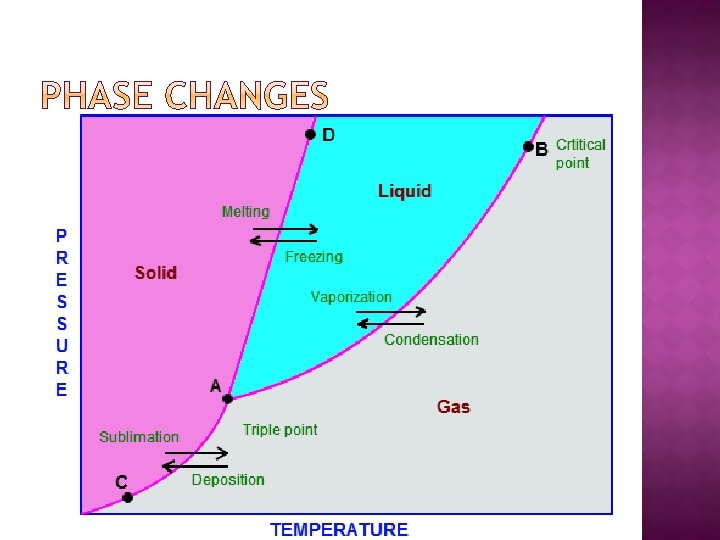
Exothermicreactions that release energy - Recombination - Deposition - Freezing Endothermicreactions that must take in energy to occur - Ionization - Sublimation - Melting


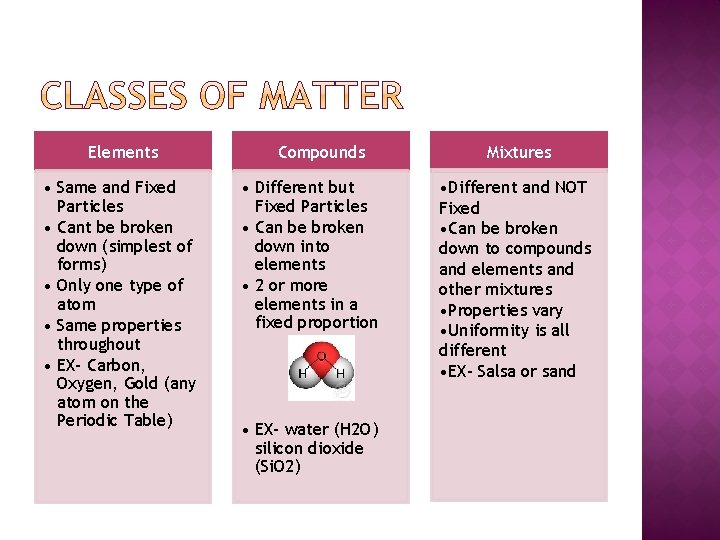
Elements • Same and Fixed Particles • Cant be broken down (simplest of forms) • Only one type of atom • Same properties throughout • EX- Carbon, Oxygen, Gold (any atom on the Periodic Table) Compounds • Different but Fixed Particles • Can be broken down into elements • 2 or more elements in a fixed proportion • EX- water (H 2 O) silicon dioxide (Si. O 2) Mixtures • Different and NOT Fixed • Can be broken down to compounds and elements and other mixtures • Properties vary • Uniformity is all different • EX- Salsa or sand

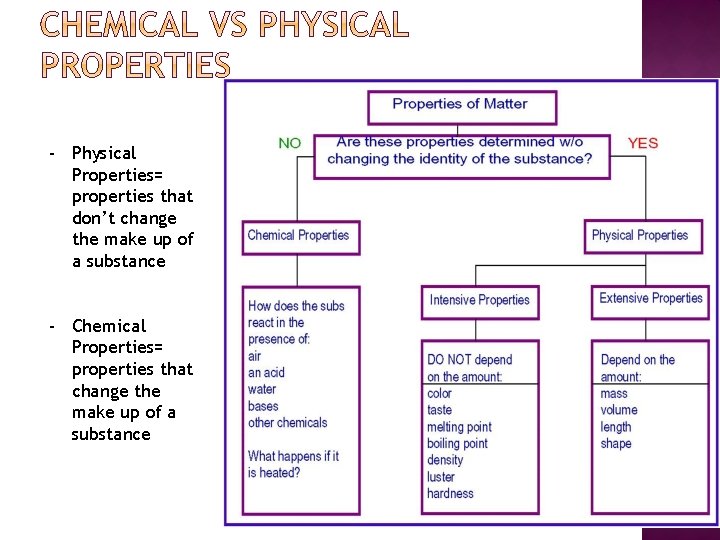
- Physical Properties= properties that don’t change the make up of a substance - Chemical Properties= properties that change the make up of a substance


� You will be turning in the following for grading: �Completed Notes �Phase Change Poster �Physical vs Chemical Properties worksheet �Root Beer Lab Write-up