Kinetic Theory Kinetic Theory What is it A








- Slides: 15

Kinetic Theory

Kinetic Theory What is it? ? ? • A theory that says that all matter is made up of tiny particles that are constantly in motion. For example……. .

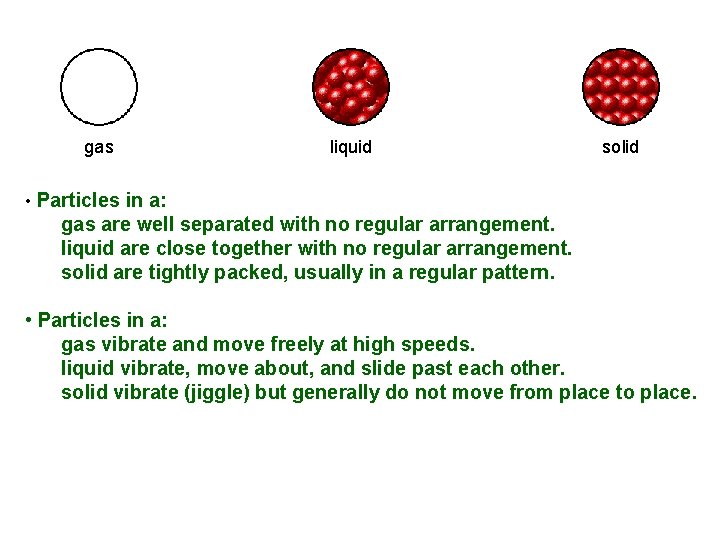
Here are three pictures that represent atoms in a solid, a liquid, and a gas. Which is which? ? ? A B C

gas liquid solid • Particles in a: gas are well separated with no regular arrangement. liquid are close together with no regular arrangement. solid are tightly packed, usually in a regular pattern. • Particles in a: gas vibrate and move freely at high speeds. liquid vibrate, move about, and slide past each other. solid vibrate (jiggle) but generally do not move from place to place.

http: //www. harcourtschool. com/activity/states_of_matter/


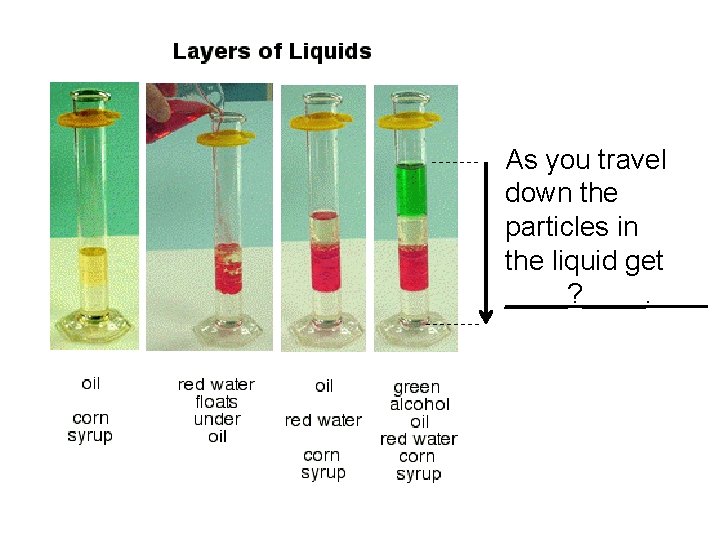
The closeness of these particles is also related to the density of a material.

As you travel down the particles in the liquid get ____? ____.

Take a look at the two boxes below. Each box has the same volume (takes up the same amount of space). If each ball has the same mass, which box would weigh more? Why?

The box that has more red balls has more mass per unit of volume. This property of matter is called density. The density of a material helps to distinguish it from other materials. Since mass is usually expressed in grams and volume in cubic centimeters (milliliters), density is expressed in grams/cubic centimeter (milliliters). We can calculate density using the formula: Density = Mass / Volume

Ever done this? ? ?

Finding volume by using Water Displacement The level before adding object The level after adding object 13 ml 14. 2 ml Volume: 14. 2 ml – 13 ml = 1. 2 ml

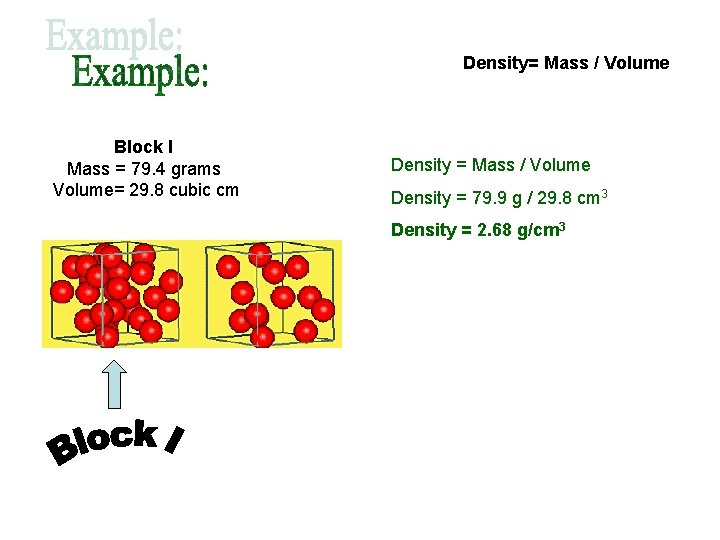
Density= Mass / Volume Block I Mass = 79. 4 grams Volume= 29. 8 cubic cm Density = Mass / Volume Density = 79. 9 g / 29. 8 cm 3 Density = 2. 68 g/cm 3

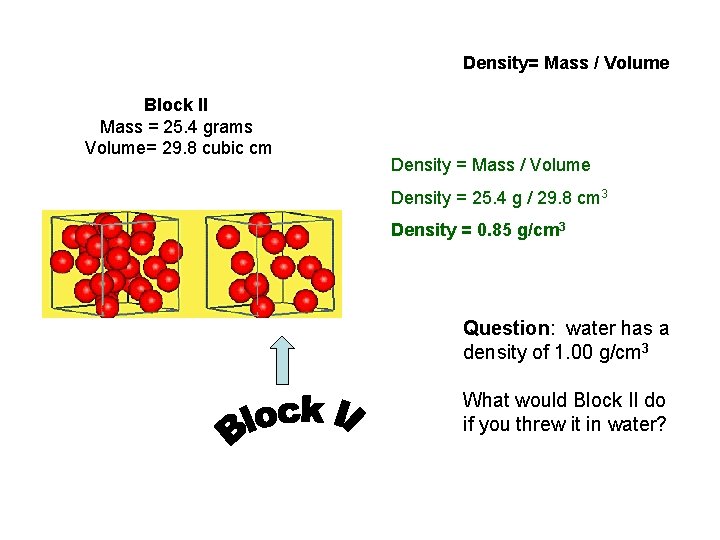
Density= Mass / Volume Block II Mass = 25. 4 grams Volume= 29. 8 cubic cm Density = Mass / Volume Density = 25. 4 g / 29. 8 cm 3 Density = 0. 85 g/cm 3 Question: water has a density of 1. 00 g/cm 3 What would Block II do if you threw it in water?

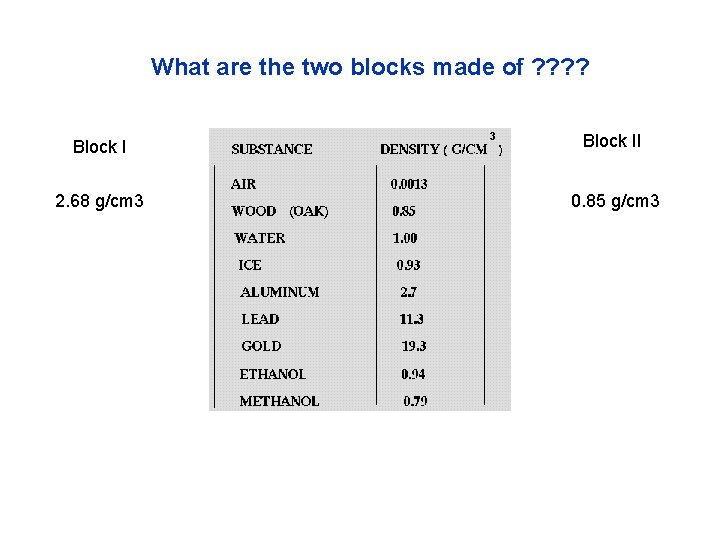
What are the two blocks made of ? ? Block II 2. 68 g/cm 3 0. 85 g/cm 3
 Chapter 14 solids liquids and gases
Chapter 14 solids liquids and gases The kinetic theory of matter states that
The kinetic theory of matter states that Kinetic theory of matter definition
Kinetic theory of matter definition Define kinetic theory of matter
Define kinetic theory of matter The kinetic theory explains how particles in matter behave
The kinetic theory explains how particles in matter behave Kinetic theory for ideal gases
Kinetic theory for ideal gases The kinetic molecular theory
The kinetic molecular theory Adhesive force
Adhesive force Kinetic molecular theory of liquids
Kinetic molecular theory of liquids Kinetic molecular theory
Kinetic molecular theory Kinetic molecular theory def
Kinetic molecular theory def Condensation particle theory
Condensation particle theory Timeline of kinetic molecular theory
Timeline of kinetic molecular theory The attraction between particles gives solids a definite
The attraction between particles gives solids a definite Kinetic theory of gases
Kinetic theory of gases Charles law in terms of kinetic molecular theory
Charles law in terms of kinetic molecular theory




























