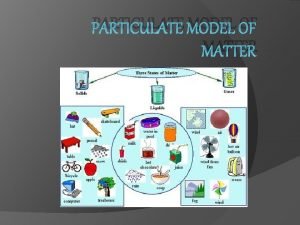
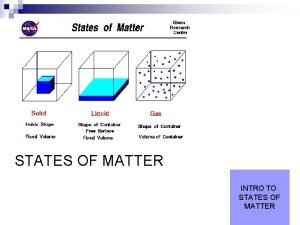
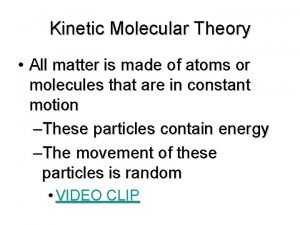
Kinetic Theory All matter is made up of
















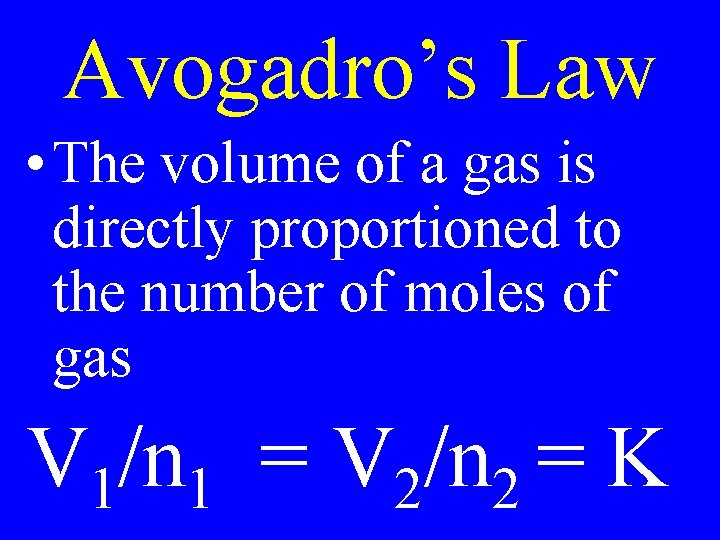














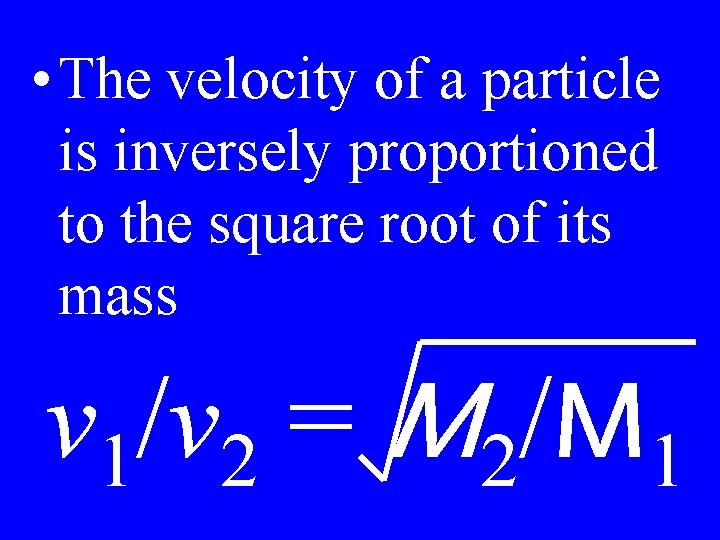


















- Slides: 54

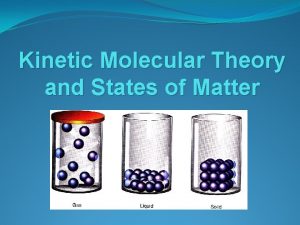
Kinetic Theory

• All matter is made up of tiny particles • The particles are in constant motion • All collisions are elastic

Pressure

• Force per unit area • Caused by collisions against a surface • Gas measured in pressure

Units of Pressure • k. Pa: kilopascal (Std Unit) • Pascal: newton/sq. meter • Atmosphere (Atm): • mm Hg:

Standard Pressure • 101. 3 k. Pa • 1. 00 Atm • 760 mm Hg or Torrs • 30. 0 inches Hg • 1013 millibars

Gas Laws

State the Following Laws • Boyle’s Law • Charles’ Law • Gay Lussac’s Law • Dalton’s Law • Graham’s Law

Boyle’s Law

• The pressure & volume of a gas at constant temperature are inversely proportioned P 1 V 1 = P 2 V 2 = K

Charles’ Law

• The volume and temperature of a gas at constant pressure are directly proportioned V 1/T 1 = V 2/T 2 = K

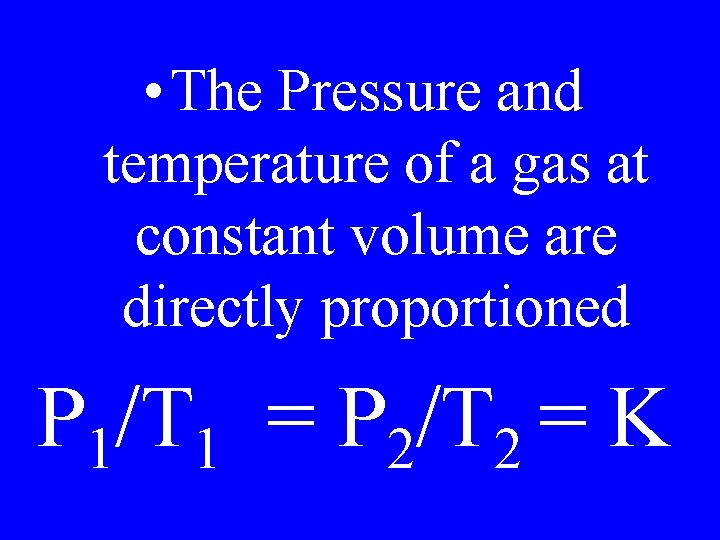
Guy Lussac’s Law

• The Pressure and temperature of a gas at constant volume are directly proportioned P 1/T 1 = P 2/T 2 = K

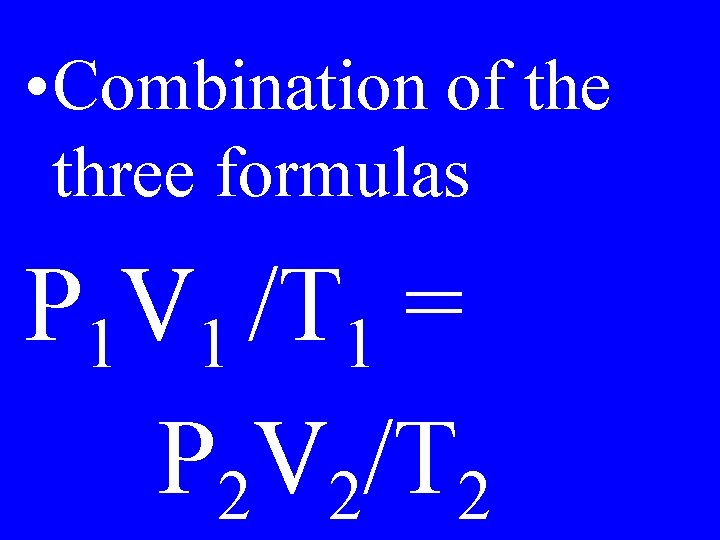
Combined Gas Law

• Combination of the three formulas P 1 V 1 /T 1 = P 2 V 2/T 2

Chm II Homework • Read Ch 12 • Work problems: 31, 32, & 33 on page 477

Calculate the new volume of 5. 0 L of gas when its pressure is doubled and its temperature is tripled:
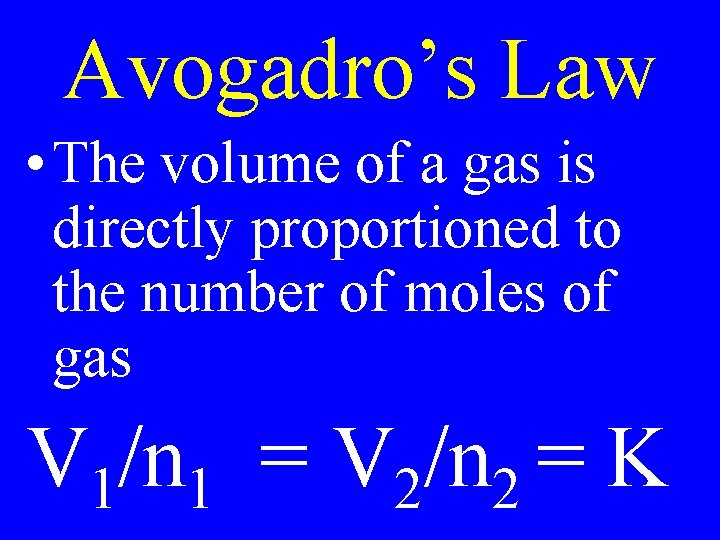
Avogadro’s Law • The volume of a gas is directly proportioned to the number of moles of gas V 1/n 1 = V 2/n 2 = K

New Combination P 1 V 1/n 1 T 1 = P 2 V 2/n 2 T 2 = K

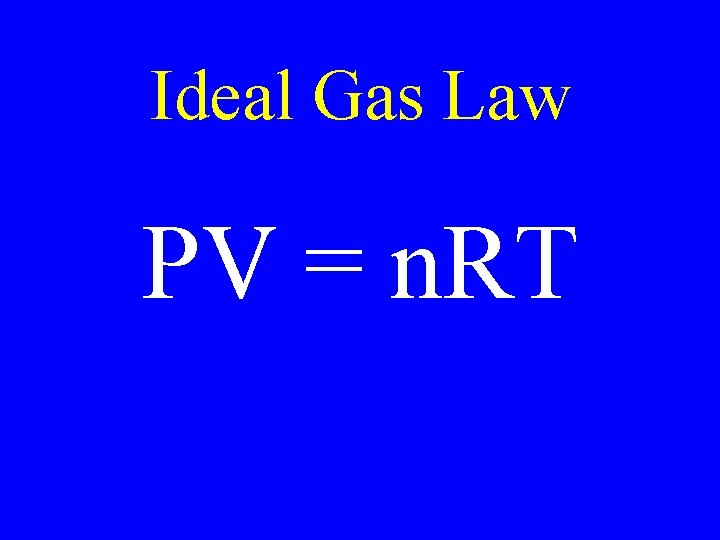
Ideal Gas Law PV = n. RT

Drill: Calculate the volume of 3. 0 moles of gas at o -23 C under 83. 1 k. Pa pressure.

Calculate the number of moles of gas occupying 831 m. L under 250 k. Pa at o 227 C

Calculate the number of moles of gas occupying 831 m. L under 80. 0 k. Pa at o 127 C

Calculate the mass of CO 2 occupying 83. 1 m. L under 25 GPa at o 227 C

Drill: Calculate the volume in m. L of 4. 0 g bromine o gas at 127 C under 83. 1 k. Pa pressure.

Ideal Gas Law PV = n. RT

Related Formulas m D or r = V m/n MW =

Calculate the molecular mass of 5. 0 g of gas occupying 831 m. L under 250 o MPa at 227 C

Chm II Homework • Read Ch 12 • Work problems: 46, 47, & 51 on page 478

Drill: Calculate the density of carbon o dioxide at 27 C under 83. 1 k. Pa pressure

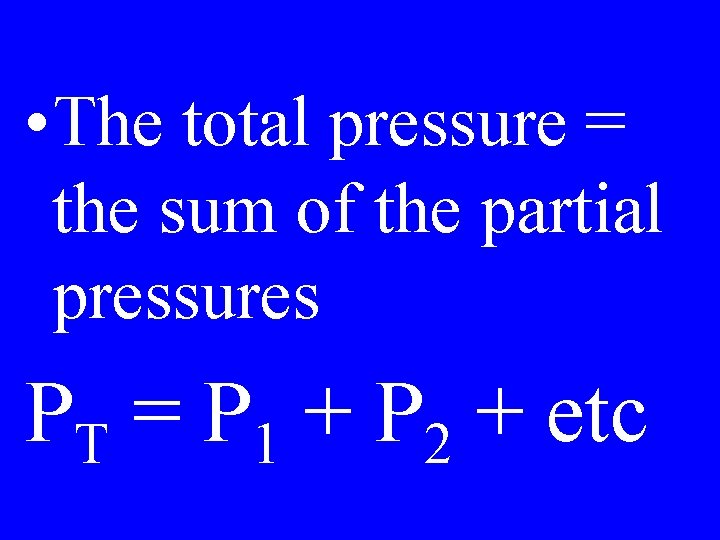
Dalton’s Law

• The total pressure = the sum of the partial pressures PT = P 1 + P 2 + etc

The total pressure of a system is 120. 0 k. Pa. The partial pressure of gas A is 112. 0 k. Pa. Determine the pressure of gas B

Graham’s Law
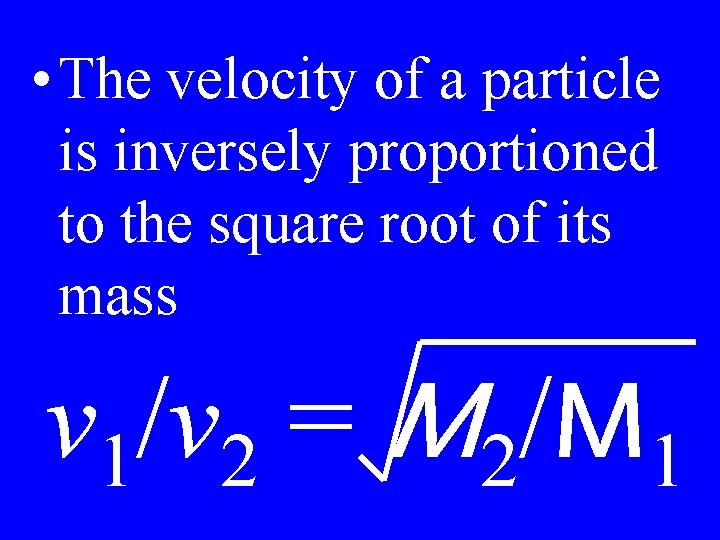
• The velocity of a particle is inversely proportioned to the square root of its mass v 1/v 2 = M 2/M 1

Chm II Classwork • Work problems: 51, & 58 on page 478

Drill: Calculate the ratio of the velocities of He gas to HCl gas:

Calculate the volume of a gas at STP when it occupies 80. 0 m. L at o 127 C under 303. 9 k. Pa pressure:

Calculate the mass of 831 m. L of CO 2 o at 27 C under 150 k. Pa pressure:

Drill: Calculate the volume of 4. 0 moles of gas under 83. 1 k. Pa o pressure at 127 C:

Calculate the volume of a gas at STP when it occupies 80. 0 m. L at o 127 C under 303. 9 k. Pa pressure:

Calculate the volume of 4. 0 moles of gas under 83. 1 k. Pa o pressure at 127 C:

Calculate the molecular mass of 50 g of gas occupying 831 m. L under 250 o MPa at 227 C

Calculate the mass of 831 m. L of CO 2 o at 167 C under 150 k. Pa pressure:

The total pressure of a system is 120. 0 k. Pa. The partial pressure of gas A is 112. 0 k. Pa. Determine the pressure of gas B

The total pressure of a system is 150. 0 k. Pa. The system contains 50 % A, 30 % B, & 20 % C. Determine the pressure of each gas.

Drill: Calculate the mass of CO 2 occupying 83. 1 m. L under 25 MPa at o 477 C

Calculate the density of carbon o dioxide at 27 C under 83. 1 k. Pa pressure

Calculate the ratio of the velocities of He gas to HCl gas:

Calculate the velocity HBr when the velocity Be is 270 m/s:

Calculate the final volume that 3. 0 L of gas will obtain when the absolute temperature is tripled & the pressure is halved.

Calculate the mass of CO occupying o 831 k. L at 227 C under 2. 50 Mpa pressure.

Calculate the volume of o H 2 formed at 27 C under 150 k. Pa when 6. 8 mg NH 3 decomposes making N 2 & H 2.
 The kinetic theory of matter states that
The kinetic theory of matter states that Kinetic theory of matter definition
Kinetic theory of matter definition Kinetic theory of matter
Kinetic theory of matter The kinetic theory explains how particles in matter behave
The kinetic theory explains how particles in matter behave Kinetic particle theory questions
Kinetic particle theory questions What is a kinetic theory of matter
What is a kinetic theory of matter All matter is made up of
All matter is made up of All matter is made up of
All matter is made up of All matter is made up of
All matter is made up of Name three lines
Name three lines Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Gray matter in the brain
Gray matter in the brain Composition of matter section 1
Composition of matter section 1 Chapter 2 section 1 classifying matter answer key
Chapter 2 section 1 classifying matter answer key What makes up the diencephalon
What makes up the diencephalon Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Gray matter and white matter
Gray matter and white matter Gray matter
Gray matter Flow of energy vs flow of matter
Flow of energy vs flow of matter Matter is made up of
Matter is made up of Everything around us is made of
Everything around us is made of Kinetic molecular theory of solid
Kinetic molecular theory of solid Kinetic molecular model of gases
Kinetic molecular model of gases Kinetic molecular theory volume
Kinetic molecular theory volume Kinetic molecular theory of liquids and solids
Kinetic molecular theory of liquids and solids Kinetic molecular theory of liquids
Kinetic molecular theory of liquids Kinetic energy molecular theory
Kinetic energy molecular theory Kinetic theory def
Kinetic theory def Kinetic molecular theory timeline
Kinetic molecular theory timeline Kinetic theory of solids
Kinetic theory of solids Kinetic theory of gases
Kinetic theory of gases Charles law in terms of kinetic molecular theory
Charles law in terms of kinetic molecular theory Postulates of kinetic theory of gas
Postulates of kinetic theory of gas Kenitic molecular theory
Kenitic molecular theory Kinetic theory of gases
Kinetic theory of gases Write postulates of kinetic theory of gases
Write postulates of kinetic theory of gases Pv=1/3nmc^2
Pv=1/3nmc^2 Write the postulates of kinetic theory of gases
Write the postulates of kinetic theory of gases Kinetic molecular theory
Kinetic molecular theory General gas equation is
General gas equation is Tenets of kinetic molecular theory
Tenets of kinetic molecular theory All matter is in constant
All matter is in constant All matter is
All matter is What is the upward force that fluids exert on all matter
What is the upward force that fluids exert on all matter Now all has been heard here is the conclusion of the matter
Now all has been heard here is the conclusion of the matter Four phase changes
Four phase changes Matter makes it all up
Matter makes it all up Is anything that has mass and volume
Is anything that has mass and volume Matter anything that takes up space
Matter anything that takes up space Intro to matter
Intro to matter All life is composed of matter
All life is composed of matter The basic unit of all matter is the
The basic unit of all matter is the Slave to myself
Slave to myself Genesis 41:52
Genesis 41:52 Nuclear fission
Nuclear fission