Kinetic Theory All matter is in constant motion







- Slides: 14

Kinetic Theory All matter is in constant motion: -atoms, ions, molecules -s, l, g, p (States of Matter) Gases: 1)Gas is made of particles -atoms/molecules -mostly empty space -weak forces between particles 2)Gas particles in constant motion -random 3)Collisions are perfectly elastic -energy transfer 100% - T, KE -all particles have same KE KE = 1/2 mv 2 constant Gas Properties http: //phet. colorado. edu/en/simulations/c ategory/chemistry

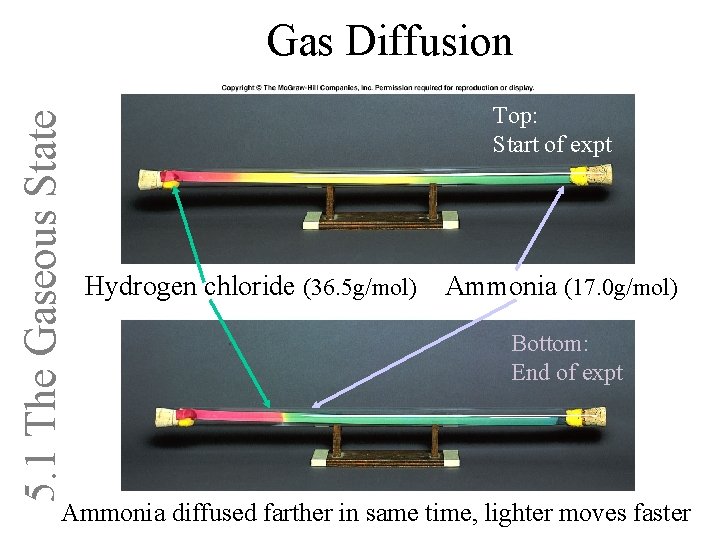
5. 1 The Gaseous State Gas Diffusion Top: Start of expt Hydrogen chloride (36. 5 g/mol) Ammonia (17. 0 g/mol) Bottom: End of expt Ammonia diffused farther in same time, lighter moves faster

-0 K = -273°C = absolute zero everything freezes no KE -Gas pressure-force created by collisions of particles on object -Atmospheric pressure-force created by collisions of particles on an object caused by gravity -Barometer-measures pressure 1 atm = 760 mm. Hg = 101. 3 k. Pa sea level -STP = 0°C, 1 atm

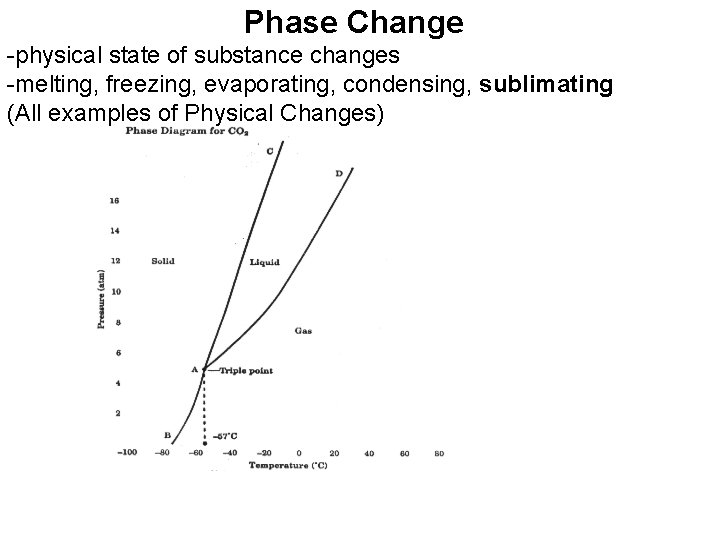
Phase Change -physical state of substance changes -melting, freezing, evaporating, condensing, sublimating (All examples of Physical Changes)

4 scientists: Dalton Robert Boyle Jacques Charles Joseph Gay-Lussac Gas Laws http: //phet. colorado. edu/en/simulations/c ategory/chemistry Real Gases-particles: volume random motion collisions elastic weak attractive forces under pressure, liquefied and solidified Ideal Gases-particles: no volume random motion collisions elastic no attractive forces under pressure, not liquefied and solidified Since real gases are mostly empty space and attractive forces are extremely weak, real gases act like ideal gases in most situations!!!

Dalton’s Law of Partial Pressures: -In a mixture of gases, the total pressure is the sum of the pressures for each gas in the mixture. PT = P 1 + P 2 + P 3 + …. V=constant T=constant ex/A scuba tank is filled with He, O 2, and N 2. The He is used since it cuts down on the risk of the diver contracting the “Bends”. How much He is in the tank if the tank is filled with 20 atm N 2, 4 atm O 2, and the total pressure is 24. 6 atm? http: //www. youtube. com/watch? v= N 5 xft 2 f. Iq. QU

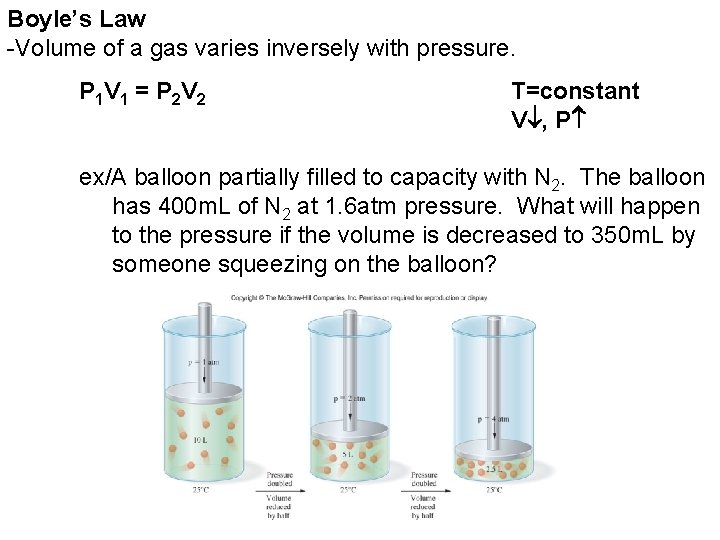
Boyle’s Law -Volume of a gas varies inversely with pressure. P 1 V 1 = P 2 V 2 T=constant V , P ex/A balloon partially filled to capacity with N 2. The balloon has 400 m. L of N 2 at 1. 6 atm pressure. What will happen to the pressure if the volume is decreased to 350 m. L by someone squeezing on the balloon?

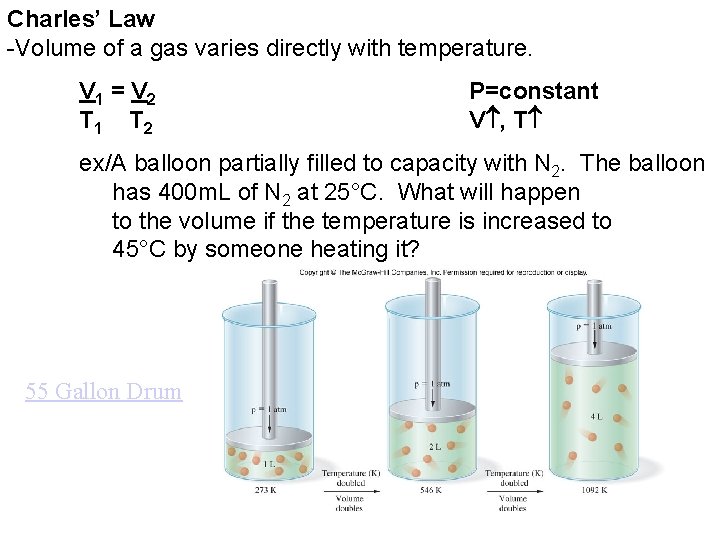
Charles’ Law -Volume of a gas varies directly with temperature. V 1 = V 2 T 1 T 2 P=constant V , T ex/A balloon partially filled to capacity with N 2. The balloon has 400 m. L of N 2 at 25°C. What will happen to the volume if the temperature is increased to 45°C by someone heating it? 55 Gallon Drum

Gay-Lussac’s Law -Pressure of a gas varies directly with temperature. P 1 = P 2 T 1 T 2 V=constant P , T T must be in K ex/A spray can of paint contains a constant volume of paint at 16 atm, 25°C. What will happen if the can is heated to 120°C? It will explode at 30 atm.

Combined Gas Law: -Ties Boyle’s, Charles’, Gay-Lussac’s Laws into one. Memorize this one!!! Others are derived from it! P 1 V 1 = P 2 V 2 T 1 T 2 T=constant. : Boyle’s P=constant. : Charles’ V=constant. : Gay-L’s ex/You go to Party City to buy your friend a mylar balloon since she is graduating. The conditions inside the store are 22°C. The balloon is filled with 5. 6 L of He at a pressure of 2 atm. You put the balloon in your car and drive home. The temp inside your car is 34°C, a hot Summer day. Since the balloon is mylar, it only expands to 5. 7 L. What is the final pressure of the balloon? What will probably happen to the balloon?

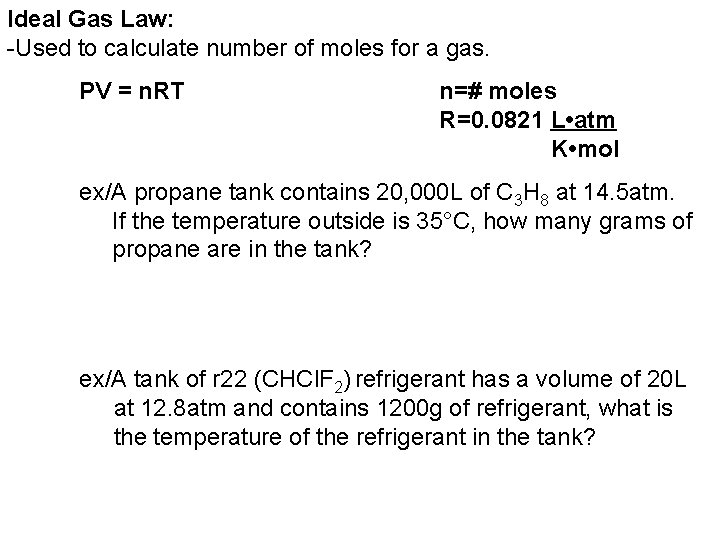
Ideal Gas Law: -Used to calculate number of moles for a gas. PV = n. RT n=# moles R=0. 0821 L • atm K • mol ex/A propane tank contains 20, 000 L of C 3 H 8 at 14. 5 atm. If the temperature outside is 35°C, how many grams of propane are in the tank? ex/A tank of r 22 (CHCl. F 2) refrigerant has a volume of 20 L at 12. 8 atm and contains 1200 g of refrigerant, what is the temperature of the refrigerant in the tank?

Liquids: -particles in motion, slide, less random -particles held together by weak attractive forces unlike gases -KE liquid < gas -Vaporization-liquid gas, below boiling pt -Evaporation-vaporization in open space - T, KE all particles. : Those closest to surface have enough energy to escape liquid. This is how energy is removed from the liquid and why temperature of the liquid remains the same as the liquid is heated further. -Dynamic Equilibrium-rate evaporation=rate condensation. : Process doesn’t stop. All particles have an equal chance to evaporate and condense. Adding more heat increases the rate of evaporation and decreases condensation! -Boiling Point-Temp which vapor pressure=atmospheric pressure. : vapor escapes from liquid -Normal Boiling Point-boiling point at 1 atm ex/bpt water: Dover=100°C Nittany Mountains=94°C

Solids: -particles packed close together in organized pattern-xtal structure -particles do not move, only vibrate/rotate -KE solid < liquid - T, KE, vibrations/rotations. : melting -Melting Point-temp solid liquid. : particles slide -no temperature increase until all solid liquid. : equilibrium between solid and liquid T, rate melting, rate solidification -Ionic Compounds- melting points since strong attraction between particles -Covalent Compounds- melting points since weaker attraction between particles -Amorphous-solid lacking xtal structure. : properties solid+liquid ex/LCD, rubber, polymers, glass

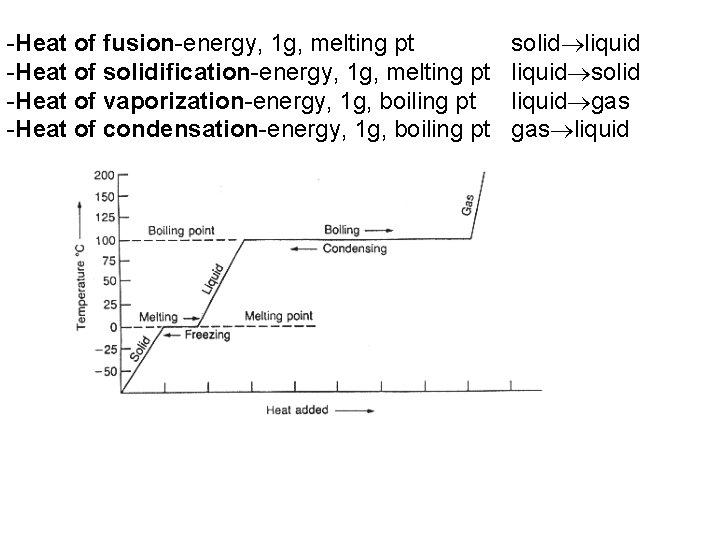
-Heat of fusion-energy, 1 g, melting pt -Heat of solidification-energy, 1 g, melting pt -Heat of vaporization-energy, 1 g, boiling pt -Heat of condensation-energy, 1 g, boiling pt solid liquid gas liquid