KINETIC MOLECULAR THEORY WITH STATES CATEGORIES OF MATTER























- Slides: 36

KINETIC MOLECULAR THEORY WITH STATES & CATEGORIES OF MATTER

KINETIC-MOLECULAR THEORY • This theory describes the behavior of gases in terms of particles in motion • Objects in motion have kinetic energy • Gases consist of small particles that are separated from one another by empty space • • • The volume of the molecules is small compared with the empty space, therefore, there are no significant attractive or repulsive forces among them The Kinetic Theory: Speed and size of particles is a reflection of temperature

MOLECULAR-KINETIC THEORY • Gas particles are in constant, random motion • They continue in one path until they will strike a substance, resulting in an elastic collision • Energy is conserved during these collisions • Energy is calculated as KE = 0. 5 m v 2 • **Kinetic energy and temperature are related • Temperature is a measure of the average kinetic energy of the particles in a sample of matter • Thus, at a given temperature, all gas molecules will have the same kinetic energy

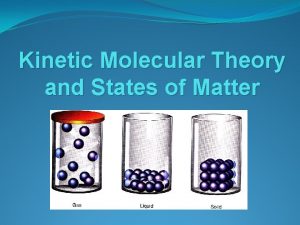
SOLID, LIQUID, GAS (a) Particles in solid (b) Particles in liquid (c) Particles in gas

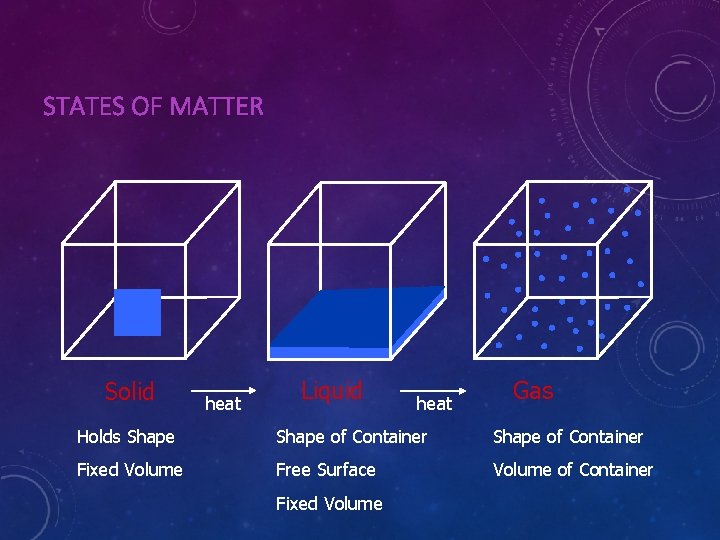
STATES OF MATTER Solid heat Liquid heat Gas Holds Shape of Container Fixed Volume Free Surface Volume of Container Fixed Volume

SOLIDS • A mole of solid particles has as much kinetic energy as a mole of liquid particles at the same temperature • Solids have stronger attractive forces • More order in solids than liquids • Density: mass / volume • Particles are more closely packed than those in a liquid, thus more dense • Some solids float. Don’t make this assumption.

LIQUIDS • Although the kinetic-molecular theory was designed for gases, it can be used for other states of matter • Liquids • Can take the shape of its containers but has a fixed volume • So it can form any shape but not expand • **They can be compressed but only slightly compared to gases because their molecules are already close together • A huge amount of force is required to condense them even by a few percent

LIQUIDS • Fluidity: ability to flow • Liquids (and gases) are classified as fluids because they can flow • Both can diffuse • Gases diffuse faster than liquids • Viscosity: measure of resistance of a liquid to flow • Liquid molecules are close enough for attractive forces to slow their movement as they pass one another • The stronger the attractive forces, the more viscosity • Size and shape of molecules also affects viscosity • Viscosity decreases with higher temperatures • The increase in temperature = increase in kinetic energy

BEHAVIOR OF GASES 1. Low Density • Density is mass / volume • The low density is not from the mass of the atoms but from the empty space (volume) between the atoms • So this means fewer atoms per volume compared to solids 2. Compression • Gases can be compressed so that the molecules will come closer to each other, like squeezing a pillow 3. Expansion • The gases will expand after the compression back to its original volume (shape) • Diffusion: the random movement of molecules through a medium, which occurs from an area of high concentration to low concentration until there is equal distance between all molecules (equilibrium) • Effusion: a process where gas escapes through a tiny opening (e. g. punctured tire) • Smaller molar mass molecules will move faster and diffuse/effuse quicker

PLASMA • Matter is superheated to such a high degree that the kinetic energy (motion or speed) of the atoms is very intense • The speeds are so fast that the nuclei speed away from their electrons and become ionized (cations!) • The positively charged nuclei are moving around in the sample and can recombine with their negative electrons if and when they slow down (electrons are still present in the “matter” but not bound to their corresponding nuclei) • Difficult to create on Earth because of the intense temperature but very abundant in stars • Stars, lightning, fluorescent/neon lights

A FIFTH PHASE OF MATTER? ? ? Bose-Einstein Condensate • What happens when a gas is cooled to absolute zero? • A new door to the quantum world opens up because all the atoms start "marching in lockstep", forming one giant matter wave • The Bose-Einstein Condensate • Atoms so close together that they move as one in a wave-like pattern instead of random motion

QUANTUM MECHANICS & QUANTUM COMPUTERS? ? ? Bose-Einstein Condensate • FYI: The coldest temperature on the scale is absolute zero • 0 K or -273 °C • This is physically impossible to reach because of disappearing matter • The atoms are so close together (but not fused) that they act as one giant atom

PHASES CHANGES • Five phases of matter: • Bose-Einstein Condensate → Solid → Liquid → Gas → Plasma • Thermal energy is transferred when going between phases • Energy is absorbed when going from left to right and energy is released when going right to left (above)

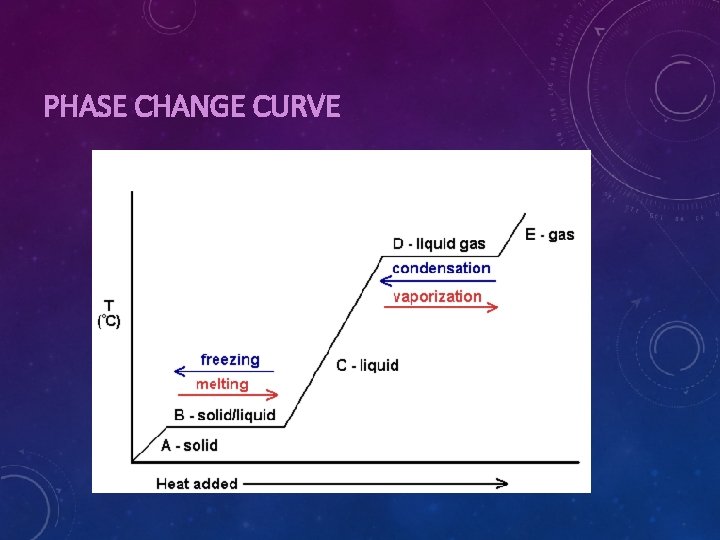
PHASE CHANGES Phases & Transitions • Melting: Solid to liquid • Freezing: Liquid to solid • Condensation: Gas to liquid • Vaporization: Liquid to gas • Sublimation: Solid to gas • Deposition: Gas to solid • Ionization: Gas to plasma • Recombination: Plasma to gas Melting/Boiling Points • At the melting point, all thermal energy is added to overcome the forces holding the particles together in solid state • This is similar to the boiling point except thermal energy is trying to overcome the liquid state • During these transitions, kinetic energy does NOT increase, thus temperature does not either

PHASE CHANGE CURVE

MOLECULAR “EVENTS” DURING PHASE CHANGES • When a sample of matter is heated from solid to liquid, the atoms begin to move faster and spread out • Increasing volume and decreasing density • The remaining part of the solid material now has less mass (equal total mass but less solid more liquid) and therefore has a dropped in temperature. • The “hot” atoms have moved away from the solid and therefore the remaining atoms are moving at slower speeds than the “hot” ones and thus average kinetic energy (i. e. temperature) is now lower!! • This is a cooling process – mind blowing concept!!! • When matter is cooled, the atoms slow down and come closer together (increase density due to decrease smaller volume) • The matter now has less kinetic energy than it did before, so where did this energy go? • It heated the environment around it as it expelled the energy outward, therefore a heating process!

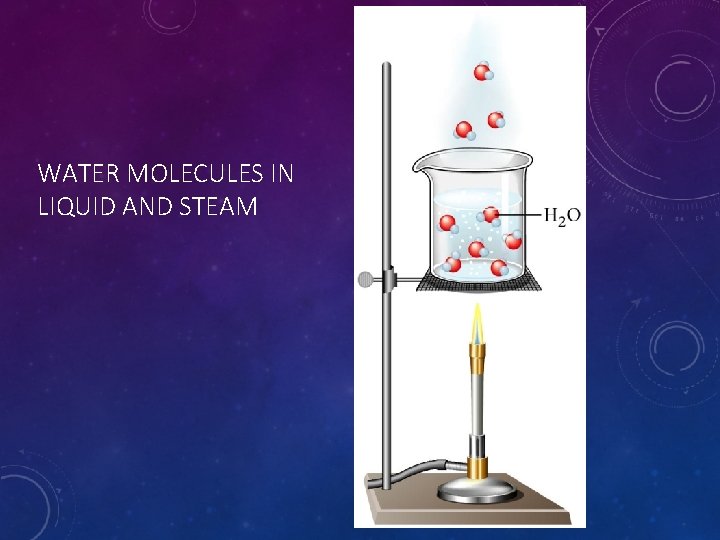
WATER MOLECULES IN LIQUID AND STEAM

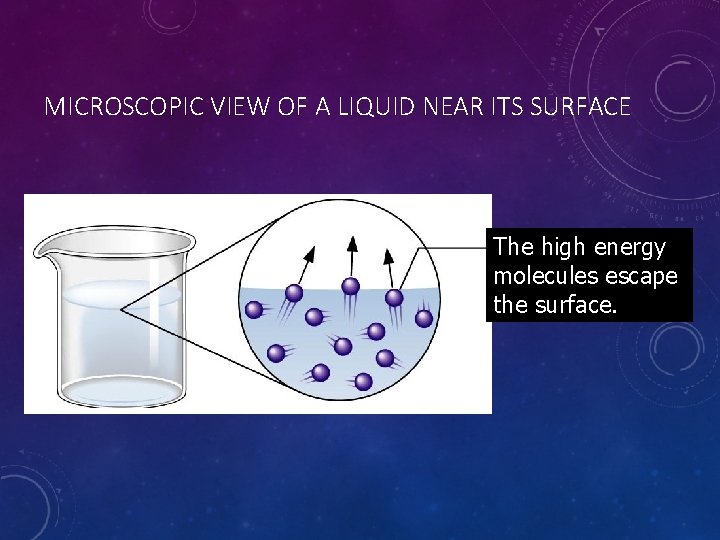
MICROSCOPIC VIEW OF A LIQUID NEAR ITS SURFACE The high energy molecules escape the surface.

PURE & IMPURE • If a material is pure, it consists of only a single element or compound • If the material is impure, then it is a mixture or contains two or more elements or compounds • Do not be fooled by labels that claim their product is pure… “pure 100% orange juice” • Mixtures are termed either heterogeneous or homogeneous

MATTER Can it be physically separated? MIXTURE PURE SUBSTANCE Is the composition uniform? Can it be chemically decomposed? Homogeneous Mixture (solution) Colloids Heterogeneous Mixture Compound Suspensions Element

PURE SUBSTANCES • Element • composed of identical atoms • e. g. - copper wire, aluminum foil

COMPOUND • Compound • composed of two or more elements in a fixed ratio • properties differ from those of individual elements • e. g. - table salt (Na. Cl)

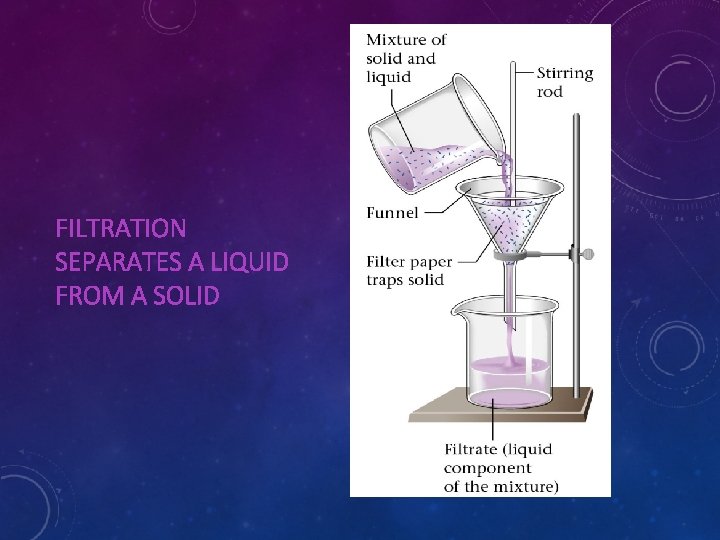
MIXTURES • A mixture is a combination of two or more substances in which each substance retains its properties • Most materials we see daily are mixtures • Mixture of elements, compounds or both • The components of a mixture can be separated from one another by taking advantage of differences in the components’ physical properties • Solids and liquids can be separated by filter paper (Filtration)

DIFFERENT MIXTURES • Remember what the prefixes mean… • Hetero: different • Homo: same • Heterogeneous mixtures are when the different components can be seen as individuals • e. g. sand in water, pulp in orange juice, salad dressings • Homogeneous mixtures have the same composition throughout, therefore any region of the mixture has the same ratio of substances

HETEROGENEOUS MIXTURES • Suspension: a mixture containing particles that settle out if left undisturbed for a period of time • These particles are larger than those found in solvated particles and gravity has greater force on them • ***Particles in a solution are atomic-scale size*** • Fine sand, silt in water or tomato juice • Colloids: • Mixtures of medium sized particles (between suspensions and solutions • Milk, fog or jello

METHODS OF SEPARATING MIXTURES • Magnet • Evaporation • Decant • Filter • Chromatography • Distillation • Centrifuge

FILTRATION SEPARATES A LIQUID FROM A SOLID


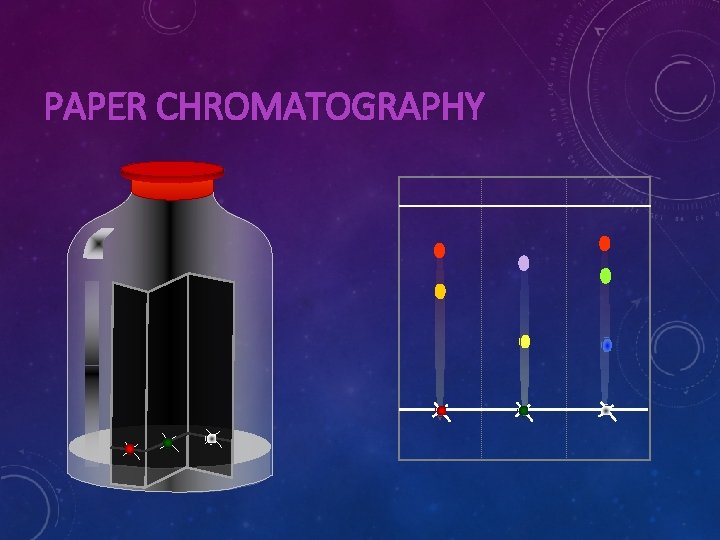
CHROMATOGRAPHY • Tie-dye t-shirt • Black pen ink • DNA testing • Tomb of Unknown Soldiers • Crime scene • Paternity testing

PAPER CHROMATOGRAPHY

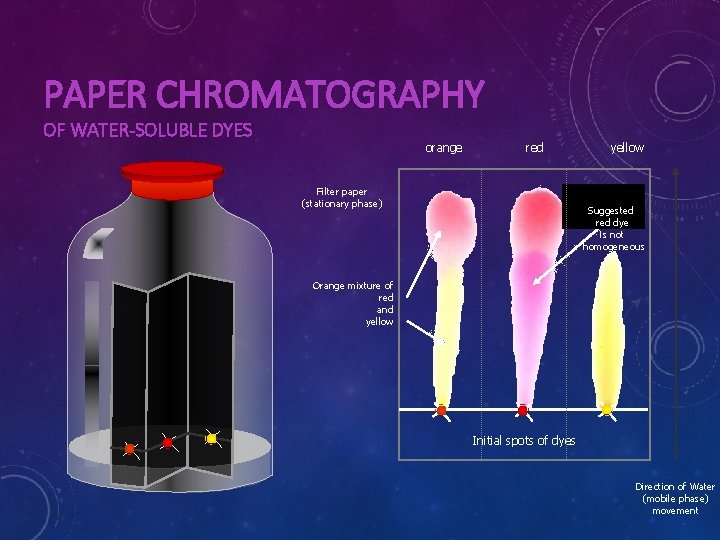
PAPER CHROMATOGRAPHY OF WATER-SOLUBLE DYES orange red Filter paper (stationary phase) yellow Suggested red dye is not homogeneous Orange mixture of red and yellow Initial spots of dyes Direction of Water (mobile phase) movement

EVAPORATION

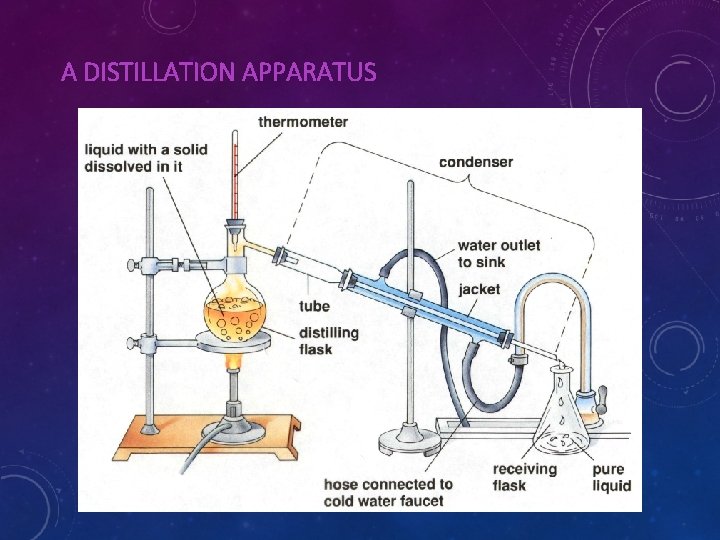
A DISTILLATION APPARATUS

THE SOLUTION IS BOILED AND STEAM IS DRIVEN OFF

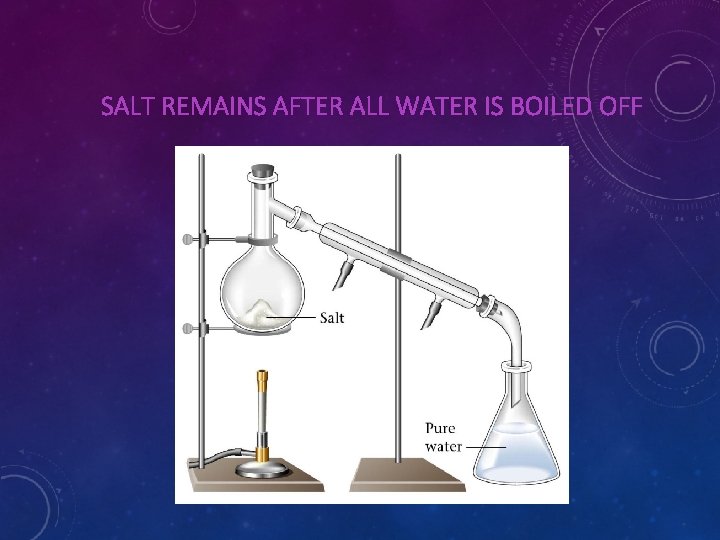
SALT REMAINS AFTER ALL WATER IS BOILED OFF

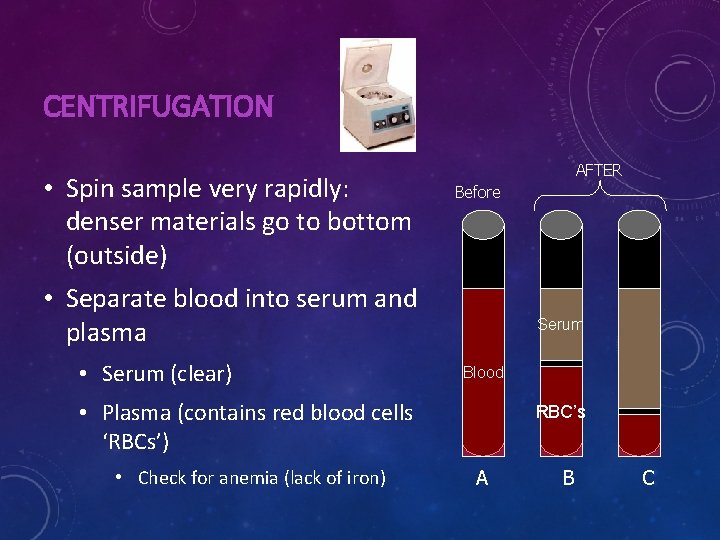
CENTRIFUGATION • Spin sample very rapidly: denser materials go to bottom (outside) AFTER Before • Separate blood into serum and plasma • Serum (clear) Serum Blood • Plasma (contains red blood cells ‘RBCs’) • Check for anemia (lack of iron) RBC’s A B C
 The kinetic theory of matter states that
The kinetic theory of matter states that Chapter 14 solids liquids and gases
Chapter 14 solids liquids and gases Kinetic molecular theory
Kinetic molecular theory Kinetic molecular theory volume
Kinetic molecular theory volume Kinetic molecular theory of liquids and solids
Kinetic molecular theory of liquids and solids Kinetic molecular theory of liquids
Kinetic molecular theory of liquids Kinetic energy molecular theory
Kinetic energy molecular theory Kinetic molecular theory def
Kinetic molecular theory def Kinetic molecular theory timeline
Kinetic molecular theory timeline Charles law in terms of kinetic molecular theory
Charles law in terms of kinetic molecular theory Postulates of kinetic molecular theory
Postulates of kinetic molecular theory Kenitic molecular theory
Kenitic molecular theory Postulates of kinetic theory of gases
Postulates of kinetic theory of gases Kinetic molecular theory formula
Kinetic molecular theory formula Kinetic molecular theory
Kinetic molecular theory Tenets of kinetic molecular theory
Tenets of kinetic molecular theory Kinetic theory of matter definition
Kinetic theory of matter definition Kinetic theory of matter
Kinetic theory of matter An explanation of how particles in matter behave
An explanation of how particles in matter behave Kinetic particle model
Kinetic particle model What is a kinetic theory of matter
What is a kinetic theory of matter Site:slidetodoc.com
Site:slidetodoc.com Pz and dxz overlap
Pz and dxz overlap Valence bond theory and molecular orbital theory
Valence bond theory and molecular orbital theory Valence bond theory and molecular orbital theory
Valence bond theory and molecular orbital theory Bond order of li2
Bond order of li2 Name two categories used to classify properties of matter
Name two categories used to classify properties of matter Covalently bonded substances
Covalently bonded substances Ionic covalent metallic
Ionic covalent metallic Zinc oxide + nitric acid → zinc nitrate + water
Zinc oxide + nitric acid → zinc nitrate + water Whats the study of matter and energy
Whats the study of matter and energy Four states of matter
Four states of matter Four states of matter
Four states of matter 5 states of matter
5 states of matter Thermal energy in states of matter
Thermal energy in states of matter Changing state
Changing state Phet states of matter basics
Phet states of matter basics