Kinetic Molecular Theory THE KINETICMOLECULAR THEORY of GASES








- Slides: 18

Kinetic Molecular Theory

THE KINETIC-MOLECULAR THEORY of GASES (the particle view) �The Kinetic Molecular Theory is used to explain the behavior of gases. �The kinetic molecular theory shows how individual gas particles interact with one another.

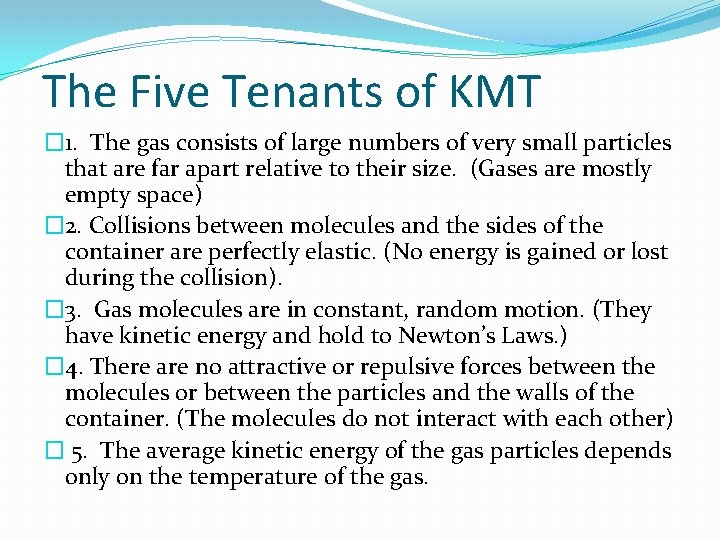
The Five Tenants of KMT � 1. The gas consists of large numbers of very small particles that are far apart relative to their size. (Gases are mostly empty space) � 2. Collisions between molecules and the sides of the container are perfectly elastic. (No energy is gained or lost during the collision). � 3. Gas molecules are in constant, random motion. (They have kinetic energy and hold to Newton’s Laws. ) � 4. There are no attractive or repulsive forces between the molecules or between the particles and the walls of the container. (The molecules do not interact with each other) � 5. The average kinetic energy of the gas particles depends only on the temperature of the gas.

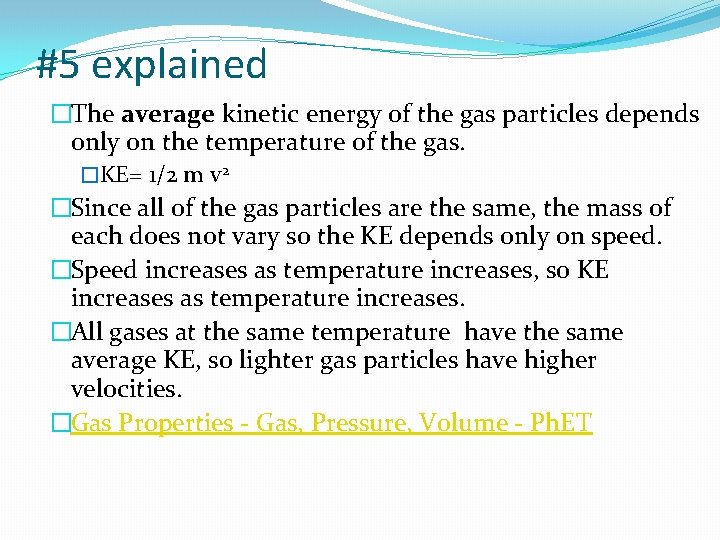
#5 explained �The average kinetic energy of the gas particles depends only on the temperature of the gas. �KE= 1/2 m v 2 �Since all of the gas particles are the same, the mass of each does not vary so the KE depends only on speed. �Speed increases as temperature increases, so KE increases as temperature increases. �All gases at the same temperature have the same average KE, so lighter gas particles have higher velocities. �Gas Properties - Gas, Pressure, Volume - Ph. ET

�To fully describe a gas four conditions must be measured: �volume �Temperature �number of molecules �Pressure

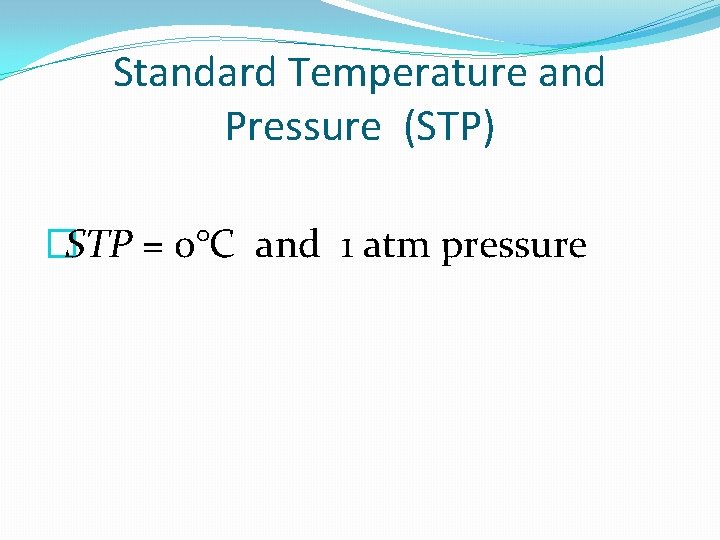
Standard Temperature and Pressure (STP) �STP = 0°C and 1 atm pressure

The Gas Laws –The macroscopic view �The mathematical relationships between pressure, temperature, volume and the amount of a gas. �Three different laws, Boyle’s Law, Charles’s Law and Gay-Lussac’s Law, are manipulated to form the Combined Gas Law.

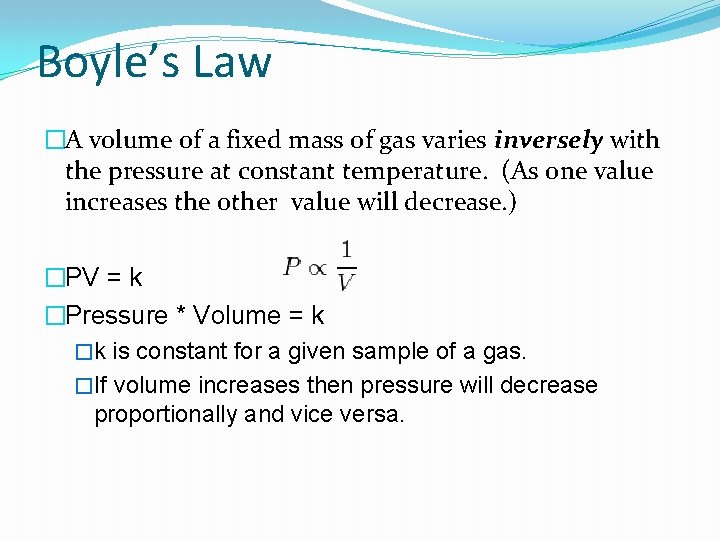
Boyle’s Law �A volume of a fixed mass of gas varies inversely with the pressure at constant temperature. (As one value increases the other value will decrease. ) �PV = k �Pressure * Volume = k �k is constant for a given sample of a gas. �If volume increases then pressure will decrease proportionally and vice versa.

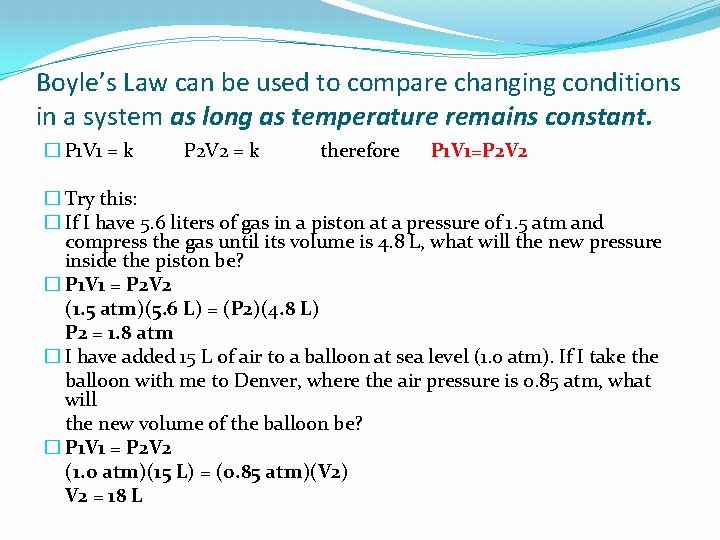
Boyle’s Law can be used to compare changing conditions in a system as long as temperature remains constant. � P 1 V 1 = k P 2 V 2 = k therefore P 1 V 1=P 2 V 2 � Try this: � If I have 5. 6 liters of gas in a piston at a pressure of 1. 5 atm and compress the gas until its volume is 4. 8 L, what will the new pressure inside the piston be? � P 1 V 1 = P 2 V 2 (1. 5 atm)(5. 6 L) = (P 2)(4. 8 L) P 2 = 1. 8 atm � I have added 15 L of air to a balloon at sea level (1. 0 atm). If I take the balloon with me to Denver, where the air pressure is 0. 85 atm, what will the new volume of the balloon be? � P 1 V 1 = P 2 V 2 (1. 0 atm)(15 L) = (0. 85 atm)(V 2) V 2 = 18 L

Charles' Law �Charles determined through his studies that when the temperature of a gas changes, the volume changes. �If the volume or temperature of the gas changes , you get the same constant. From this, Charles came up with this statement: V 1/T 1 = V 2/T 2 �Temperature, needs to be given in Kelvin. Why?

�If we have 2. 00 L of methane gas at a temperature of 40. 0° Celsius, what will the volume be if we heat the gas to 80. 0 ° Celsius? �The first thing we have to do is convert the temperatures to Kelvins (by adding 273). �The initial temperature is 40. 0 + 273 = 313 K �The final temperature is 80. 0 + 273 = 353 K. �V 1/T 1 = V 2/T 2 � 2 L / 313 K = V 2 / 353 K �V 2 = 2. 26 L

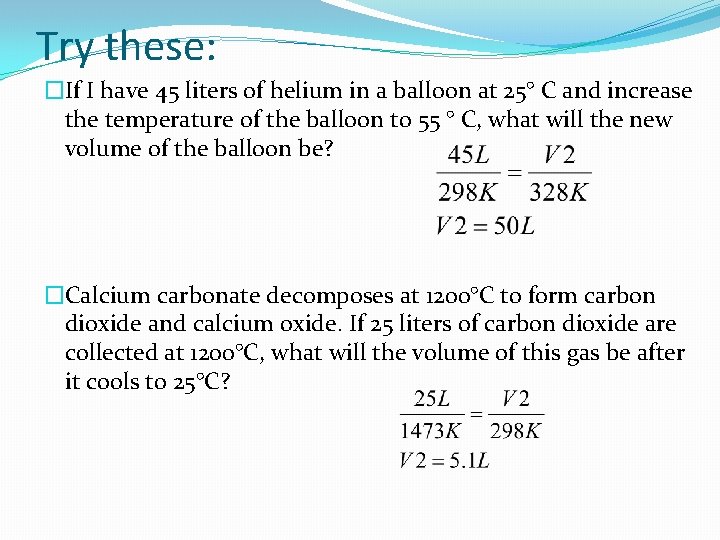
Try these: �If I have 45 liters of helium in a balloon at 25° C and increase the temperature of the balloon to 55 ° C, what will the new volume of the balloon be? �Calcium carbonate decomposes at 1200°C to form carbon dioxide and calcium oxide. If 25 liters of carbon dioxide are collected at 1200°C, what will the volume of this gas be after it cools to 25°C?

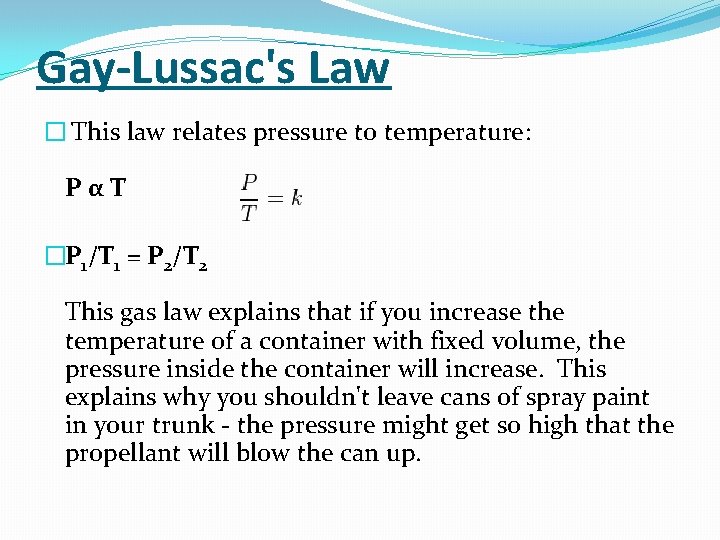
Gay-Lussac's Law � This law relates pressure to temperature: PαT �P 1/T 1 = P 2/T 2 This gas law explains that if you increase the temperature of a container with fixed volume, the pressure inside the container will increase. This explains why you shouldn't leave cans of spray paint in your trunk - the pressure might get so high that the propellant will blow the can up.

Try these: �A cylinder contain a gas which has a pressure of 125 k. Pa at a temperature of 200 K. Find the temperature of the gas which has a pressure of 100 k. Pa. �T 2 = 160 K �Find the final pressure of gas at 150 K, if the pressure of gas is 210. k. Pa at 120 K. �P 2 = 262. k. Pa

The Combined Gas Law �The combined gas law combines the previous laws. �(P 1 V 1) / T 1 = (P 2 V 2) / T 2 �In this equation, all of the terms are exactly the same as in the preceding equations. �Whenever you're changing the conditions of pressure, volume, and/or temperature for a gas, you just plug the numbers into this equation. �If one of these variables isn't mentioned in the problem, just ignore it entirely. �Suppose that the temperature of the gas didn't change while you were making your change. Since the first temperature term and the second are the same, they cancel out.

Example: �If we have two liters of a gas at a temperature of 420 K and decrease the temperature to 350 K, what will the new volume of the gas be? �To solve this problem, use the combined gas law to find the answer. Since pressure was never mentioned in this problem, just ignore it. As a result, the equation will be: �V 1/T 1 = V 2/T 2 Charles's law

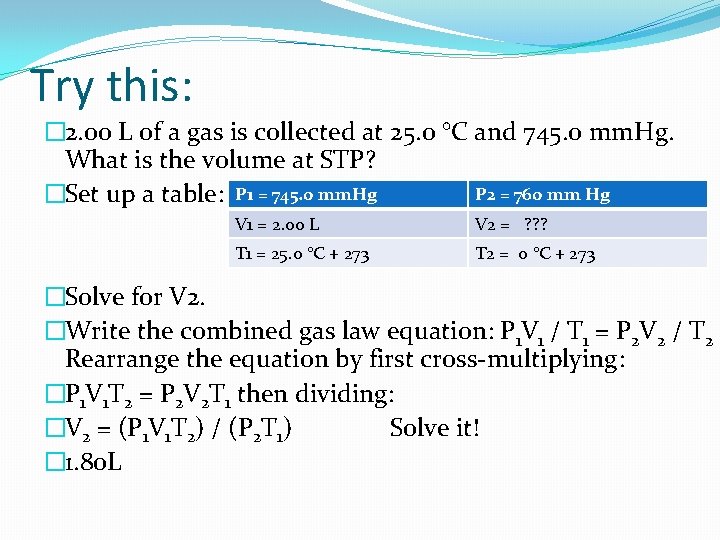
Try this: � 2. 00 L of a gas is collected at 25. 0 °C and 745. 0 mm. Hg. What is the volume at STP? P 2 = 760 mm Hg �Set up a table: P 1 = 745. 0 mm. Hg V 1 = 2. 00 L V 2 = ? ? ? T 1 = 25. 0 °C + 273 T 2 = 0 °C + 273 �Solve for V 2. �Write the combined gas law equation: P 1 V 1 / T 1 = P 2 V 2 / T 2 Rearrange the equation by first cross-multiplying: �P 1 V 1 T 2 = P 2 V 2 T 1 then dividing: �V 2 = (P 1 V 1 T 2) / (P 2 T 1) Solve it! � 1. 80 L

Home Fun �Practice Problems– The Gas Laws